Difference between revisions of "Borax" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 83: | Line 83: | ||
== Chemistry == | == Chemistry == | ||
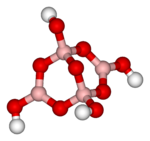
| − | [[Image:Tetraborate-ion-3D-vdW.png|left|150px|thumb|The structure of the anion [B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]<sup>2−</ | + | [[Image:Tetraborate-ion-3D-vdW.png|left|150px|thumb|The structure of the anion [B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]<sup>2−</sup> in borax]] |
The term ''borax'' is often used for a number of closely related minerals or chemical compounds that differ in their [[Water of crystallization|crystal water]] content: | The term ''borax'' is often used for a number of closely related minerals or chemical compounds that differ in their [[Water of crystallization|crystal water]] content: | ||
| Line 91: | Line 91: | ||
* Borax decahydrate (Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O) | * Borax decahydrate (Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O) | ||
| − | Borax is generally described as Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O. However, it is better formulated as Na<sub>2</sub>[B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]·8H<sub>2</sub>O, since borax contains the [B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]<sup>2−</ | + | Borax is generally described as Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·10H<sub>2</sub>O. However, it is better formulated as Na<sub>2</sub>[B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]·8H<sub>2</sub>O, since borax contains the [B<sub>4</sub>O<sub>5</sub>(OH)<sub>4</sub>]<sup>2−</sup> ion. In this structure, there are two four-coordinate boron atoms (two BO<sub>4</sub> tetrahedra) and two three-coordinate boron atoms (two BO<sub>3</sub> triangles). |
Borax is also easily converted to [[boric acid]] and other [[borates]], which have many applications. If left exposed to dry air, it slowly loses its [[hydrate|water of hydration]] and becomes the white and chalky [[mineral]] [[tincalconite]] (Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·5H<sub>2</sub>O). | Borax is also easily converted to [[boric acid]] and other [[borates]], which have many applications. If left exposed to dry air, it slowly loses its [[hydrate|water of hydration]] and becomes the white and chalky [[mineral]] [[tincalconite]] (Na<sub>2</sub>B<sub>4</sub>O<sub>7</sub>·5H<sub>2</sub>O). | ||
| Line 97: | Line 97: | ||
When borax is burned, it produces a bright orange-colored flame. Because of this, it is sometimes used for homemade pyrotechnics. | When borax is burned, it produces a bright orange-colored flame. Because of this, it is sometimes used for homemade pyrotechnics. | ||
| + | == See also == | ||
| − | + | * [[Boron]] | |
* [[Buffer solution]] | * [[Buffer solution]] | ||
| + | * [[Mineral]] | ||
* [[Borax bead test]] | * [[Borax bead test]] | ||
* [[Sodium borohydride]] | * [[Sodium borohydride]] | ||
* [[Ulexite]] | * [[Ulexite]] | ||
| − | + | ||
| − | + | == Notes == | |
| − | + | <references/> | |
== References == | == References == | ||
| − | + | <<Need 3 refs>> | |
| − | < | ||
== External links == | == External links == | ||
| Line 119: | Line 120: | ||
* [http://www.sefsc.noaa.gov/HTMLdocs/SodiumBorate.htm Sodium Borate in sefsc.noaa.gov] | * [http://www.sefsc.noaa.gov/HTMLdocs/SodiumBorate.htm Sodium Borate in sefsc.noaa.gov] | ||
| − | + | [[Category:Physical sciences]] | |
| − | + | [[Category:Earth sciences]] | |
| − | + | [[Category:Geology]] | |
| − | + | [[Category:Minerals]] | |
| − | |||
| − | |||
| − | |||
| − | [[Category: | ||
| − | [[Category: | ||
| − | [[Category: | ||
| − | [[Category: | ||
| − | + | {{credit|129357915}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 02:37, 9 May 2007
- For other uses, see Borax (disambiguation).
| Borax | |
|---|---|

| |
| General | |
| Systematic name | Sodium tetraborate
decahydrate |
| Molecular formula | Na2B4O7·10H2O |
| Molar mass | 381.37 g/mol |
| Appearance | white solid |
| CAS number | 1303-96-4 |
| Properties | |
| Density and phase | 1.73 g/cm³, solid |
| Solubility in water | 5.1 g/100 ml (20 °C) |
| Melting point | 75 °C |
| Boiling point | 320 °C |
| Basicity (pKb) | see text |
| Structure | |
| Coordination geometry |
? |
| Crystal structure | Monoclinic |
| Thermodynamic data | |
| Std enthalpy of formation ΔfH |
-3276.75 kJ/mol |
| Standard molar entropy S |
189.53 J·K−1·mol−1 |
| Hazards | |
| MSDS | External MSDS |
| EU classification | not listed |
| NFPA 704 | |
| Flash point | non-flammable |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Sodium aluminate
Sodium gallate |
| Other cations | Potassium tetraborate |
| Related compounds | Boric acid
Sodium perborate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Borax, also called sodium borate, or sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.
Borax has a wide variety of uses. It is a component of many detergents, cosmetics, and enamel glazes. It is also used to make buffer solutions in biochemistry, as a fire retardant, as an anti-fungal compound for fibreglass, as an insecticide, as a flux in metallurgy, and as a precursor for other boron compounds.
The term borax is used for a number of closely related minerals or chemical compounds that differ in their crystal water content, but usually refers to the decahydrate. Commercially sold borax is usually partially dehydrated.
Name
The origin of the name is traceable to the Medieval Latin borax, which comes from the Arabic buraq, which comes from either the Persian burah [1] or the Middle Persian burak[2].
Uses
Buffer
Sodium borate is used in biochemical and chemical laboratories to make buffer solutions, e.g. for gel electrophoresis of DNA. It has a lower conductivity, produces sharper bands, and can be run at higher speeds than can gels made from TBE Buffer or TAE Buffer (5 - 35 V/cm as compared to 5 - 10 V/cm). At a given voltage, the heat generation and thus the gel temperature is much lower than with TBE or TAE buffers, therefore the voltage can be increased to speed up electrophoresis so that a gel run takes only a fraction of the usual time. Downstream applications, such as isolation of DNA from a gel slice or Southern blot analysis, work as expected with sodium borate gels. Borate buffers (usually at pH 8) are also used as preferential equilibration solution in DMP-based crosslinking reactions.
Lithium borate is similar to sodium borate and has all of its advantages, but permits use of even higher voltages due to the lower conductivity of lithium ions as compared to sodium ions.[1] However, lithium borate is much more expensive.
Flux
A mixture of borax and ammonium chloride is used as a flux when welding iron and steel. It lowers the melting point of the unwanted iron oxide (scale), allowing it to run off. Borax is also used mixed with water as a flux when soldering jewelry metals such as gold or silver. It allows the molten solder to flow evenly over the joint in question. Borax is also a good flux for 'pre-tinning' tungsten with zinc - making the tungsten soft-solderable.[2]
Food additive
Borax is used as a food additive in some countries with the E number E285, but is banned in the United States. Its use is similar to salt, and it appears in French and Iranian caviar.
Other uses
- component of detergents
- component of cosmetics
- ingredient in enamel glazes
- component of glass, pottery, and ceramics
- fire retardant
- anti-fungal compound for fibreglass and cellulose insulation
- component of Slime
- insecticide to kill ants and fleas
- precursor for sodium perborate monohydrate that is used in detergents, as well as for boric acid and other borates
- treatment for thrush in horse's hoofs
- used to make indelible ink for dip pens by dissolving shellac into heated borax
Natural sources
Borax occurs naturally in evaporite deposits produced by the repeated evaporation of seasonal lakes (see playa). The most commercially important deposits are found in Turkey and near Boron, California and other locations in the Southwestern United States, the Atacama desert in Chile, and in Tibet. Borax can also be produced synthetically from other boron compounds.
Toxicity
Boric acid, sodium borate, and sodium perborate are estimated to have a fatal dose from 0.1 to 0.5g/kg.[3] These substances are toxic to all cells, and have a slow excretion rate through the kidneys. Kidney toxicity is the greatest, with liver fatty degeneration, cerebral edema, and gastroenteritis. Boric acid solutions used as an eye wash or on abraded skin are known to be especially toxic to infants, especially after repeated use due to its slow elimination rate.[4]
Chemistry
The term borax is often used for a number of closely related minerals or chemical compounds that differ in their crystal water content:
- Anhydrous borax (Na2B4O7)
- Borax pentahydrate (Na2B4O7·5H2O)
- Borax decahydrate (Na2B4O7·10H2O)
Borax is generally described as Na2B4O7·10H2O. However, it is better formulated as Na2[B4O5(OH)4]·8H2O, since borax contains the [B4O5(OH)4]2− ion. In this structure, there are two four-coordinate boron atoms (two BO4 tetrahedra) and two three-coordinate boron atoms (two BO3 triangles).
Borax is also easily converted to boric acid and other borates, which have many applications. If left exposed to dry air, it slowly loses its water of hydration and becomes the white and chalky mineral tincalconite (Na2B4O7·5H2O).
When borax is burned, it produces a bright orange-colored flame. Because of this, it is sometimes used for homemade pyrotechnics.
See also
- Boron
- Buffer solution
- Mineral
- Borax bead test
- Sodium borohydride
- Ulexite
Notes
- ↑ Digital object identifier (DOI): 10.1016/j.ab.2004.05.054 Analytical Biochemistry 2004; 333: 1-13
- ↑ Digital object identifier (DOI): 10.1119/1.1972398 Am. J. Phys. 34, xvi (1966)
- ↑ Handbook of Poisoning, Robert H. Dreisback, eighth edition, p.314
- ↑ Goodman and Gillman's: The Pharmacological Basis of Therapeutics, 6th edition, chapter on Antiseptics and Disinfectants, page 971
ReferencesISBN links support NWE through referral fees
<<Need 3 refs>>
External links
- International Chemical Safety Card 0567
- International Chemical Safety Card 1229 (fused borax)
- National Pollutant Inventory - Boron and compounds
- NIOSH Pocket Guide to Chemical Hazards
- Sodium Borate in sefsc.noaa.gov
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.