Difference between revisions of "Azo compound" - New World Encyclopedia
(added credit and category tags, deleted foreign language links) |
|||
| Line 16: | Line 16: | ||
== See also == | == See also == | ||
| + | |||
| + | * [[Diazonium compound]] | ||
* [[Azo coupling]] | * [[Azo coupling]] | ||
* [[Ponceau 4R]] | * [[Ponceau 4R]] | ||
* [[Ponceau S]] | * [[Ponceau S]] | ||
| + | |||
| + | == Notes == | ||
| + | <references/> | ||
==References== | ==References== | ||
| − | + | ||
| + | == External links == | ||
| + | |||
{{Functional Groups}} | {{Functional Groups}} | ||
| Line 28: | Line 35: | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
[[Category:Organic chemistry]] | [[Category:Organic chemistry]] | ||
| − | |||
| − | |||
{{credit|260468986}} | {{credit|260468986}} | ||
Revision as of 17:31, 7 January 2009
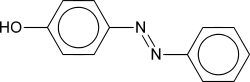
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. The N=N group is called an azo group, although the parent compound, HNNH, is called diimide. The more stable derivatives contain two aryl groups. The name azo comes from azote, the French name of nitrogen that is derived from the Greek a (not) + zoe (to live).
As dyes and pigments
As a consequence of п-delocalization, aryl azo compounds have vivid colours, especially reds, oranges, and yellows. Therefore, they are used as dyes, azo dyes for example Disperse Orange 1. Some azo compounds, eg. methyl orange, are used as acid-base indicators due to the different colours of their acid and salt forms. The development of azo dyes was an important step in the development of the chemical industry.
Azo pigments are colorless particles (typically earths or clays), which have been colored using an azo compound. Azo pigments are important in a variety of paints including artist's paints. They have excellent coloring properties, again mainly in the yellow to red range, as well as lightfastness. The lightfastness depends not only on the properties of the organic azo compound, but also on the way they have been adsorbed on the pigment carrier. Azo pigments are advantageous because they are non-toxic.
Organic chemistry
Aryl azo compounds
Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the trans isomer, but upon photolysis, converts to the cis isomer. Aromatic azo compounds can be synthesized by using an azo coupling reaction, which entails an electrophilic substitution reaction where a aryl diazonium cation attacks another aryl ring, especially those substituted with electron-releasing groups.[1] Since diazonium salts are often unstable near room temperature, the azo coupling reactions are typically conducted near ice temperatures. The oxidation of hydrazines (R-NH-NH-R') also gives azo compounds.[2]
Alkyl azo compounds
Aliphatic azo compounds (R and/or R' = aliphatic) are less commonly encountered than the aryl azo compounds. One example is diethyldiazene, EtN=NEt.[3] At elevated temperatures or upon irradiation, the carbon-nitrogen (C-N) bonds in certain alkyl azo compounds cleave with the loss of nitrogen gas to generate radicals. Owing to this process, some aliphatic azo compounds are utilized as radical initiators. Representative is Azobisisobutylonitrile (AIBN) which is widely used as an initiator in polymerization. Because of their instability, especially for aliphatic ones, care should be taken with the handling of azo compounds or an explosion may occur.
See also
- Diazonium compound
- Azo coupling
- Ponceau 4R
- Ponceau S
Notes
- ↑ H. T. Clarke and W. R. Kirner (1941). "Methyl Red". Org. Synth.; Coll. Vol. 1: 374.
- ↑ March, J. “Advanced Organic Chemistry” $th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- ↑ Ohme, R.; Preuschhof, H.; Heyne, H.-U. (1988). "Azoethane". Org. Synth.; Coll. Vol. 6: 78.
ReferencesISBN links support NWE through referral fees
External links
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.