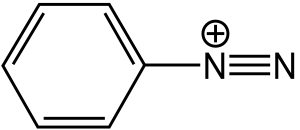
Diazonium compound
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group with the characteristic structure of R-N2+ X- where R can be any organic residue such alkyl or aryl and X is an inorganic or organic anion such as a halogen. Historically, diazonium salts have been developed as important intermediates in the organic synthesis of dyes.
Preparation
The process of forming diazoniums compound is called diazotation, diazoniatin, or diazotization. The reaction was discovered by Peter Griess in 1858, who subsequently discovered several reactions of the new compound.
The most important method for the preparation of diazonium salts is treatment of aromatic amines such as aniline with sodium nitrite in the presence of a mineral acid. In aqueous solution these salts are unstable at temperatures higher than +5 °C; the -N+≡N group tends to be lost as N2, i.e. nitrogen gas. One can isolate diazonium compounds as tetrafluoroborate salts, which are stable at room temperature. Typically diazonium compounds are not isolated and once prepared, used immediately in further reactions.
Reactions
- The most important aromatic diazonium salt reactions are azo coupling with anilines and phenols to azo compounds (azo dyes) in electrophilic aromatic substitution.
- Nitrogen replacement reactions by halogens take place in nucleophilic aromatic substitution such as the Sandmeyer Reaction, the Gomberg-Bachmann reaction and the Schiemann reaction. In the so-called Craig method, 2-aminopyridine reacts with sodium nitrite, hydrobromic acid and excess bromine to 2-bromopyridine [1]
- In Meerwein arylation the salt also decomposes and the aryl residue reacts with an electron-deficient alkene in an addition reaction
- In the Bamberger triazine synthesis and the Widman-Stoermer synthesis a diazonium salt reacts as an electrophile through its terminal nitrogen atom with an activated double bond.
- Hydrolysis of diazonium salts yields alcohols
- Reduction with hypophosphorous acid replaces the nitrogen by hydrogen, which allows amino and nitro groups to be removed easily from rings
Applications
The first use of diazonium salts was to produce water-fast dyed fabrics by immersing the fabric in an aqueous solution of the diazonium compound, then a solution of the coupler.
Diazonium salts are light sensitive and break down under near UV or violet light. This property has led to their use in document reproduction. In this process, paper or film is coated with a diazonium salt. After contact exposure under light, the residual diazo is converted to a stable azo dye with an aqueous solution of coupler. A more common process uses a paper coated with diazo, coupler and an acid to inhibit coupling; after exposure the image is developed by a vapor mixture of ammonia and water which forces coupling.
In nanotechnology
In a nanotechnology application of diazonium salts, 4-chlorobenzenediazonium tetrafluoroborate is very efficient in functionalizing single wall nanotubes [2].
In order to exfoliate the nanotubes, they are mixed with an ionic liquid in a mortar and pestle. The diazonium salt is added together with potassium carbonate, and after 15 minutes of grinding at room temperature the surface of the nanotubes are covered with chlorophenyl groups with an efficiency of 1 in 44 carbon atoms. These added subsituents prevent the tubes from forming intimate bundles due to large cohesive forces between them which is a recurring problem in nanotube technology.
It is also possible to functionalize silicon wafers with diazonium salts forming an aryl monolayer. In one study,[3] the silicon surface is washed with ammonium hydrogen fluoride leaving it covered with silicon-hydrogen bonds (hydride passivation). The reaction of the surface with a solution of diazonium salt in acetonitrile for two hours in the dark is a spontaneous process through a free radical mechanism[4]:
The grafting of diazonium salts on metals has been accomplished on iron, cobalt, nickel, platinum, palladium, zinc, copper and gold surfaces. One interesting question raised is the actual positioning on the aryl group on the surface. An in silico study[5] demonstrates that in the period 4 elements from titanium to copper the binding energy decreases from left to right because the number of d-electrons increases. The metals to the left of iron are positioned tilted towards or flat on the surface favoring metal to carbon pi bond formation and those on the right of iron are positioned in an upright position, favoring metal to carbon sigma bond formation. This also explains why diazonium salt grafting thus far has been possible with those metals to right of iron in the periodic table.
See also
Notes
- ↑ Lyman C. Craig (1934). A Study of the Preparation of Alpha-Pyridyl Halides from Alpha-Aminopyridine by the Diazo Reaction. J. Am. Chem. Soc. 56 (1): 231-232.
- ↑ Green Chemical Functionalization of Single-Walled Carbon Nanotubes in Ionic Liquids B. Katherine Price, Jared L. Hudson, and James M. Tour J. Am. Chem. Soc.; 2005; 127(42): 14867-14870. Digital object identifier (DOI): 10.1021/ja053998c
- ↑ Direct Covalent Grafting of Conjugated Molecules onto Si, GaAs, and Pd Surfaces from Aryldiazonium Salts Michael P. Stewart, Francisco Maya, Dmitry V. Kosynkin, Shawn M. Dirk, Joshua J. Stapleton, Christine L. McGuiness, David L. Allara, and James M. Tour J. Am. Chem. Soc.; 2004; 126(1): 370-378. Digital object identifier (DOI): 10.1021/ja0383120
- ↑ Reaction sequence: silicon surface reaction with ammonium hydrogen fluoride creates hydride layer. An electron is transferred from the silicon surface to the diazonium salt in an open circuit potential reduction leaving a silicon radical cation and a diazonium radical. In the next step a proton and a nitrogen molecule are expelled and the two radical residues recombine creating a surface silicon to carbon bond.
- ↑ Structure and Bonding between an Aryl Group and Metal Surfaces De-en Jiang, Bobby G. Sumpter, and Sheng Dai J. Am. Chem. Soc.; 2006; 128(18): 6030-6031. Digital object identifier (DOI): 10.1021/ja061439f
ReferencesISBN links support NWE through referral fees
- March, Jerry. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 4th ed. New York: Wiley, 1992. ISBN 0471601802
- McMurry, John. Organic Chemistry, 6th edition. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th edition. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.