Difference between revisions of "Alanine" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 13: | Line 13: | ||
==Structure== | ==Structure== | ||
| + | In [[biochemistry]], the term "amino acid" is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these proteinogenic alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. The exception to this basic structure is [[proline]], whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. | ||
| + | |||
The [[alpha carbon|α-carbon]] atom of alanine is bound with a methyl group (-CH<sub>3</sub>), making it one of the simplest α-amino acids with respect to molecular structure and also resulting in alanine being classified as an [[aliphatic compound|aliphatic]] amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function. | The [[alpha carbon|α-carbon]] atom of alanine is bound with a methyl group (-CH<sub>3</sub>), making it one of the simplest α-amino acids with respect to molecular structure and also resulting in alanine being classified as an [[aliphatic compound|aliphatic]] amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function. | ||
| Line 20: | Line 31: | ||
==Sources== | ==Sources== | ||
Any protein-containing food such as meat, poultry, fish, eggs or dairy products is rich in alanine. [[Racemic]] alanine can be prepared via the addition of [[hydrogen cyanide]] and [[ammonia]] to [[acetaldehyde]] by the [[Strecker amino acid synthesis|Strecker reaction]].<ref> Kendall, E. C.; McKenzie, B. F. “dl-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941).http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf</ref> | Any protein-containing food such as meat, poultry, fish, eggs or dairy products is rich in alanine. [[Racemic]] alanine can be prepared via the addition of [[hydrogen cyanide]] and [[ammonia]] to [[acetaldehyde]] by the [[Strecker amino acid synthesis|Strecker reaction]].<ref> Kendall, E. C.; McKenzie, B. F. “dl-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941).http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf</ref> | ||
| + | |||
| + | ==Silk== | ||
| + | a general trend in spider silk structure is a sequence of amino acids (usually alternating [[glycine]] and [[alanine]], or alanine alone) that [[Molecular self-assembly|self-assemble]] into a [[beta sheet]] conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form [[crystals]], whereas the other segments form [[amorphous]] domains. It is the interplay between the hard crystalline segments, and the elastic semi amorphous regions, that gives spider silk its extraordinary properties. | ||
| Line 35: | Line 49: | ||
[[Category:Proteinogenic amino acids]] | [[Category:Proteinogenic amino acids]] | ||
[[Category:Glucogenic amino acids]] | [[Category:Glucogenic amino acids]] | ||
| − | {{credit|137172474}} | + | {{credit|Alanine|137172474|Spider_silk|137474753}} |
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
Revision as of 01:17, 15 June 2007
 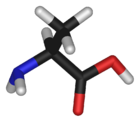
Chemical structure of L-alanine | |
Alanine | |
| Systematic (IUPAC) name | |
| (S)-2-aminopropanoic acid | |
| Identifiers | |
| CAS number | 56-41-7 |
| PubChem | 5950 |
| Chemical data | |
| Formula | C3H7NO2 |
| Mol. weight | 89.1 |
| SMILES | C[C@H](N)C(O)=O |
| Complete data | |
Alanine is an α-amino acid with the chemical formula HO2CCH(NH2)CH3. The L-isomer is one of the 20 proteinogenic amino acids, i.e. the building blocks of proteins. Its three letter code is ala, its one letter code is A, and its codons are GCU, GCC, GCA, and GCG.[1] It is classified as an nonpolar amino acid. L-alanine is second only to leucine, accounting for 7.8% of the primary structure in a sample of 1,150 proteins Template:Ref N. D-alanine occurs in bacterial cell walls and in some peptide antibiotics.
Structure
In biochemistry, the term "amino acid" is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these proteinogenic alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins.
The α-carbon atom of alanine is bound with a methyl group (-CH3), making it one of the simplest α-amino acids with respect to molecular structure and also resulting in alanine being classified as an aliphatic amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function.
Biosynthesis
Alanine is most commonly produced by reductive amination of pyruvate. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle. It also arises together with lactate and generate glucose from protein via the alanine cycle.
Sources
Any protein-containing food such as meat, poultry, fish, eggs or dairy products is rich in alanine. Racemic alanine can be prepared via the addition of hydrogen cyanide and ammonia to acetaldehyde by the Strecker reaction.[2]
Silk
a general trend in spider silk structure is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form amorphous domains. It is the interplay between the hard crystalline segments, and the elastic semi amorphous regions, that gives spider silk its extraordinary properties.
ReferencesISBN links support NWE through referral fees
- Template:Note N Doolittle RF (1989). "Redundancies in protein sequences" in Prediction of Protein Structures and the Principles of Protein Conformation. (Fasman GD, ed.), pp 599-623, Plenum Press, New York.
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Nomenclature and Symbolism for Amino Acids and Peptides. Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
- ↑ Kendall, E. C.; McKenzie, B. F. “dl-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941).http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.