Difference between revisions of "Alanine" - New World Encyclopedia
Rick Swarts (talk | contribs) (→Silk) |
Rosie Tanabe (talk | contribs) |
||
| (6 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Paid}}{{Approved}}{{Images OK}}{{Submitted}} |
{{NatOrganicBox | {{NatOrganicBox | ||
| image= [[Image:L-alanine-skeletal.svg|150px]] [[Image:L-alanine-3D-sticks.png|140px]] | | image= [[Image:L-alanine-skeletal.svg|150px]] [[Image:L-alanine-3D-sticks.png|140px]] | ||
| Line 10: | Line 10: | ||
| mass=89.1 | | mass=89.1 | ||
}} | }} | ||
| − | '''Alanine''' | + | '''Alanine''' is an one of the simplest [[amino acid]]s in terms of molecular structure and one of the most widely found in [[protein]]. In [[human being|humans]], the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be [[amino acid#essential amino acid|non-essential]] since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. It has the [[chemical formula]] HO<sub>2</sub>CCH(NH<sub>2</sub>)CH<sub>3</sub>. |
| − | Alanine is classified as | + | Alanine is classified as a nonpolar amino acid. L-alanine is second only to [[leucine]], accounting for 7.8 percent of the primary structure in a sample of 1,150 proteins (Doolittle 1989). Davidson (2007) reports that it averages about nine percent of average protein composition on a per mole basis. Alanine is also involved in the metabolism of [[tryptophan]] and the vitamin [[pyridoxine]]. |
| + | {{toc}} | ||
| + | Alanine's unique structure makes it one of the principal components of [[silk]], along with [[glycine]], providing the unique characteristics of this natural protein [[fiber]]. [[Spider]] silk is so strong that it has been said that a circular web, similar in all ways to that found in nature but the size of a [[American football|football]] field, could stop a [[airliner|commercial jetliner]] in mid-flight (Henke 2007), and yet it is so light that a single strand long enough to circle the earth would weigh less than 16 ounces (460 grams). The particular arrangement of the amino acids reveals the complex coordination in nature, a harmony that has existed for millions of years and which scientists now are studying in hope of learning how to create such a strong and yet [[elasticity|elastic]] fiber. | ||
The isomer D-alanine occurs in [[bacteria]]l cell walls and in some peptide [[antibiotic]]s. | The isomer D-alanine occurs in [[bacteria]]l cell walls and in some peptide [[antibiotic]]s. | ||
| Line 28: | Line 30: | ||
where ''R'' represents a ''side chain'' specific to each amino acid. The exception to this basic structure is [[proline]], whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group. | where ''R'' represents a ''side chain'' specific to each amino acid. The exception to this basic structure is [[proline]], whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group. | ||
| − | Most amino acids occur in two possible optical isomers, called D and L. | + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. |
In alanine, the α-carbon atom is bound with a levorotatory methyl group (-CH<sub>3</sub>), making it one of the simplest α-amino acids with respect to molecular structure (Davidson 2007)and also resulting in alanine being classified as an [[aliphatic compound|aliphatic]] amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function. | In alanine, the α-carbon atom is bound with a levorotatory methyl group (-CH<sub>3</sub>), making it one of the simplest α-amino acids with respect to molecular structure (Davidson 2007)and also resulting in alanine being classified as an [[aliphatic compound|aliphatic]] amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function. | ||
==Sources and biosynthesis== | ==Sources and biosynthesis== | ||
| − | Any protein-containing food such as meat, poultry, [[fish]], eggs or dairy products is rich in alanine. | + | Any protein-containing [[food]]—such as meat, poultry, [[fish]], eggs, or dairy products—is rich in alanine. [[Racemic]] alanine (equal amounts of left- and right-handed stereoisomers) can be prepared via the addition of [[hydrogen cyanide]] and [[ammonia]] to [[acetaldehyde]] by the Strecker reaction (Kandall and McKenzie 1941). |
Alanine is most commonly produced in the body by [[reductive amination]] (conversion of a carbonyl group to an amine) of [[pyruvate]]. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as [[glycolysis]], [[gluconeogenesis]], and the [[citric acid cycle]]. | Alanine is most commonly produced in the body by [[reductive amination]] (conversion of a carbonyl group to an amine) of [[pyruvate]]. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as [[glycolysis]], [[gluconeogenesis]], and the [[citric acid cycle]]. | ||
| Line 42: | Line 44: | ||
Alanine is a key component in [[spider]] [[silk]]. Spider silk is a remarkably strong material, with a tensile strength is comparable to that of high-grade [[steel]] (Shao and Vollrath 2002). | Alanine is a key component in [[spider]] [[silk]]. Spider silk is a remarkably strong material, with a tensile strength is comparable to that of high-grade [[steel]] (Shao and Vollrath 2002). | ||
| − | Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of alanine (Ala) and [[glycine]] (Gly), with the remaining components mostly the amino acids [[proline]] (Pro), [[tyrosine]] (Tyr), [[arginine]] (Arg), [[glutamine]] (Gln), [[serine]] (Ser), and [[leucine]] (Leu) (UB 2007). Spidroin 1 and 2 are made up of polyalanine regions with about 4 to 9 alanine monomers in a block (Beek et al. report approximately 8 monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (Beek et al. 2002; UB 2007). | + | Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of alanine (Ala) and [[glycine]] (Gly), with the remaining components mostly the amino acids [[proline]] (Pro), [[tyrosine]] (Tyr), [[arginine]] (Arg), [[glutamine]] (Gln), [[serine]] (Ser), and [[leucine]] (Leu) (UB 2007). Spidroin 1 and 2 are made up of polyalanine regions with about 4 to 9 alanine monomers in a block (van Beek et al. report approximately 8 monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (van Beek, et al. 2002; UB 2007). |
| − | The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form | + | The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form [[crystal]]s, whereas the other segments form [[amorphous]] domains. It is the interplay between the hard crystalline segments, and the elastic semi-amorphous regions, which gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007). |
| − | The glycine-rich regions give spider silk its elasticity, as each sequence of five amino acids is followed by a 180 degree turn, resulting in a spiral. Capture silk is the most elastic, with about 43 repeats on average, and can extend | + | The [[glycine]]-rich regions give spider silk its [[elasticity]], as each sequence of five amino acids is followed by a 180 degree turn, resulting in a [[spiral]]. Capture silk is the most elastic, with about 43 repeats on average, and can extend two to four times its original length, while dragline silk only repeats about nine times and can extend about 30 percent of original length (UB 2007). |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Doolittle, R. F. 1989. “Redundancies in protein sequences.” In ''Prediction of Protein Structures and the Principles of Protein Conformation''. Edited by G. D. Fasman. New York: Plenum Press. ISBN 0306431319 | |
| + | * Henke, B. 2007. [http://www.poststar.com/articles/2007/06/10/sports/columns/outdoors-bhenke/74c4533dc34bd13d852572f6000217fc.txt “The webs they weave.”] ''Poststar.com'' (June 10, 2007). Retrieved June 15, 2007. | ||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid “Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology.”] IUPAC-IUB. Retrieved June 14, 2007. | ||
| + | * Kendall, E. C., and B. F. McKenzie. 1941. [http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf dl-“Alanine.”] ''Organic Syntheses'' 1: 21. | ||
| + | * Shao, Z., and F. Vollrath. 2002. “Surprising strength of silkworm silk.” ''Nature'' 418: 741. | ||
| + | * University of Bristol, School of Chemistry (UB). 2007. [http://www.chm.bris.ac.uk/motm/spider/page3.htm “Spider silk: Chemical structure.”] University of Bristol. Retrieved June 14, 2007. | ||
| + | * van Beek, J. D., S. Hess, F. Vollrath, and B. H. Meier. 2002. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=124902 “The molecular structure of spider dragline silk: Folding and orientation of the protein backbone.”] ''Proc. Natl. Acad. Sci. USA'' 99 (16): 10266-10271. Retrieved June 14, 2007. | ||
{{AminoAcids}} | {{AminoAcids}} | ||
| − | |||
| − | |||
{{credit|Alanine|137172474|Spider_silk|137474753|Alanine_cycle|123826360}} | {{credit|Alanine|137172474|Spider_silk|137474753|Alanine_cycle|123826360}} | ||
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
| + | [[Category:Biochemistry]] | ||
| + | [[Category:Amino acids]] | ||
Latest revision as of 23:12, 15 February 2019
 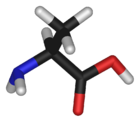
Chemical structure of L-alanine | |
Alanine | |
| Systematic (IUPAC) name | |
| (S)-2-aminopropanoic acid | |
| Identifiers | |
| CAS number | 56-41-7 |
| PubChem | 5950 |
| Chemical data | |
| Formula | C3H7NO2 |
| Mol. weight | 89.1 |
| SMILES | C[C@H](N)C(O)=O |
| Complete data | |
Alanine is an one of the simplest amino acids in terms of molecular structure and one of the most widely found in protein. In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be non-essential since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. It has the chemical formula HO2CCH(NH2)CH3.
Alanine is classified as a nonpolar amino acid. L-alanine is second only to leucine, accounting for 7.8 percent of the primary structure in a sample of 1,150 proteins (Doolittle 1989). Davidson (2007) reports that it averages about nine percent of average protein composition on a per mole basis. Alanine is also involved in the metabolism of tryptophan and the vitamin pyridoxine.
Alanine's unique structure makes it one of the principal components of silk, along with glycine, providing the unique characteristics of this natural protein fiber. Spider silk is so strong that it has been said that a circular web, similar in all ways to that found in nature but the size of a football field, could stop a commercial jetliner in mid-flight (Henke 2007), and yet it is so light that a single strand long enough to circle the earth would weigh less than 16 ounces (460 grams). The particular arrangement of the amino acids reveals the complex coordination in nature, a harmony that has existed for millions of years and which scientists now are studying in hope of learning how to create such a strong and yet elastic fiber.
The isomer D-alanine occurs in bacterial cell walls and in some peptide antibiotics.
Alanine's three letter code is ala, its one letter code is A, and its codons are GCU, GCC, GCA, and GCG (IUPAC-IUB 1983).
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis.
In alanine, the α-carbon atom is bound with a levorotatory methyl group (-CH3), making it one of the simplest α-amino acids with respect to molecular structure (Davidson 2007)and also resulting in alanine being classified as an aliphatic amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function.
Sources and biosynthesis
Any protein-containing food—such as meat, poultry, fish, eggs, or dairy products—is rich in alanine. Racemic alanine (equal amounts of left- and right-handed stereoisomers) can be prepared via the addition of hydrogen cyanide and ammonia to acetaldehyde by the Strecker reaction (Kandall and McKenzie 1941).
Alanine is most commonly produced in the body by reductive amination (conversion of a carbonyl group to an amine) of pyruvate. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle.
Alanine also arises together with lactate from protein via the alanine cycle. Thus, when muscles produce lactate during times of decreased oxygen, they also produce alanine. This alanine is shuttled to the liver where it is used to make glucose.
Silk
Alanine is a key component in spider silk. Spider silk is a remarkably strong material, with a tensile strength is comparable to that of high-grade steel (Shao and Vollrath 2002).
Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of alanine (Ala) and glycine (Gly), with the remaining components mostly the amino acids proline (Pro), tyrosine (Tyr), arginine (Arg), glutamine (Gln), serine (Ser), and leucine (Leu) (UB 2007). Spidroin 1 and 2 are made up of polyalanine regions with about 4 to 9 alanine monomers in a block (van Beek et al. report approximately 8 monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (van Beek, et al. 2002; UB 2007).
The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form amorphous domains. It is the interplay between the hard crystalline segments, and the elastic semi-amorphous regions, which gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007).
The glycine-rich regions give spider silk its elasticity, as each sequence of five amino acids is followed by a 180 degree turn, resulting in a spiral. Capture silk is the most elastic, with about 43 repeats on average, and can extend two to four times its original length, while dragline silk only repeats about nine times and can extend about 30 percent of original length (UB 2007).
ReferencesISBN links support NWE through referral fees
- Doolittle, R. F. 1989. “Redundancies in protein sequences.” In Prediction of Protein Structures and the Principles of Protein Conformation. Edited by G. D. Fasman. New York: Plenum Press. ISBN 0306431319
- Henke, B. 2007. “The webs they weave.” Poststar.com (June 10, 2007). Retrieved June 15, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. “Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology.” IUPAC-IUB. Retrieved June 14, 2007.
- Kendall, E. C., and B. F. McKenzie. 1941. dl-“Alanine.” Organic Syntheses 1: 21.
- Shao, Z., and F. Vollrath. 2002. “Surprising strength of silkworm silk.” Nature 418: 741.
- University of Bristol, School of Chemistry (UB). 2007. “Spider silk: Chemical structure.” University of Bristol. Retrieved June 14, 2007.
- van Beek, J. D., S. Hess, F. Vollrath, and B. H. Meier. 2002. “The molecular structure of spider dragline silk: Folding and orientation of the protein backbone.” Proc. Natl. Acad. Sci. USA 99 (16): 10266-10271. Retrieved June 14, 2007.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.