Difference between revisions of "Sulfur dioxide" - New World Encyclopedia
Andy Wilhelm (talk | contribs) |
Andy Wilhelm (talk | contribs) m (Protected "Sulfur dioxide": Copyedited [edit=sysop:move=sysop]) |
||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Claimed}}{{Images OK}}{{Submitted}}{{Approved}}{{Contracted}}{{Paid}} |
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! {{chembox header}} | Sulfur dioxide | ! {{chembox header}} | Sulfur dioxide | ||
| Line 117: | Line 117: | ||
|} | |} | ||
| − | '''Sulfur dioxide''' (also '''sulphur dioxide''') is the [[chemical compound]] with the formula SO<sub>2</sub>. This important gas is the main product from the combustion of [[sulfur]] compounds and is of significant environmental concern. | + | '''Sulfur dioxide''' (also '''sulphur dioxide''') is the [[chemical compound]] with the formula SO<sub>2</sub>. This important gas is the main product from the combustion of [[sulfur]] compounds and is of significant environmental concern. SO<sub>2</sub> is often described as the "smell of burning sulfur" but is ''not'' responsible for the [[hydrogen sulfide|smell of rotten eggs]]. |
| − | SO<sub>2</sub> is produced by [[volcano]]es and in various industrial processes. Since [[coal]] and [[petroleum]] contain various amounts of sulfur compounds, their combustion generates sulfur dioxide. | + | SO<sub>2</sub> is produced by [[volcano]]es and in various industrial processes. Since [[coal]] and [[petroleum]] contain various amounts of sulfur compounds, their combustion generates sulfur dioxide. Further oxidation of SO<sub>2</sub>, usually in the presence of a catalyst such as [[nitrogen dioxide|NO<sub>2</sub>]], forms [[sulfuric acid|H<sub>2</sub>SO<sub>4</sub>]], and thus [[acid rain]].<ref>Dr. Mike Thompson, |
| − | Winchester College, UK | + | Winchester College, UK. [http://www.chm.bris.ac.uk/motm/so2/so2h.htm Online Link] Retrieved December 20, 2007.</ref> |
== Preparation == | == Preparation == | ||
| Line 143: | Line 143: | ||
SO<sub>2</sub> is a bent molecule with C<sub>2v</sub> [[symmetry point group]]. | SO<sub>2</sub> is a bent molecule with C<sub>2v</sub> [[symmetry point group]]. | ||
| − | In terms of [[electron counting|electron-counting]] formalisms, the sulfur atom has an [[oxidation state]] of +4, a [[formal charge]] of | + | In terms of [[electron counting|electron-counting]] formalisms, the sulfur atom has an [[oxidation state]] of +4, a [[formal charge]] of zero, and is surrounded by five [[electron pair]]s. From the perspective of [[molecular orbital theory]], most of these electron pairs are non-bonding in character, as is typical for [[hypervalent molecule]]s. |
One conventional covalent bond is present between each oxygen and the central sulfur atom, with two further electrons delocalised between the oxygens and the sulfur atom. | One conventional covalent bond is present between each oxygen and the central sulfur atom, with two further electrons delocalised between the oxygens and the sulfur atom. | ||
== Uses == | == Uses == | ||
| − | Sulfur dioxide is sometimes used as a [[preservative]] ([[E number]]: E220<ref>[http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist#h_3 Current EU approved additives and their E Numbers] | + | Sulfur dioxide is sometimes used as a [[preservative]] ([[E number]]: E220<ref>[http://www.food.gov.uk/safereating/chemsafe/additivesbranch/enumberlist#h_3 Current EU approved additives and their E Numbers] The Food Standards Agency website. Retrieved December 20, 2007.</ref>) in alcoholic drinks,<ref>"All wines contain sulphur dioxide in various forms, collectively known as sulphites. Even in completely unsulphured wine it is present at concentrations of up to ten milligrams per litre." [http://www.morethanorganic.com/sulphur-in-the-bottle Sulphites in wine] MoreThanOrganic.com. Retrieved December 20, 2007.</ref> or dried [[apricot]]s and other [[dried fruit]]s due to its [[antimicrobial]] properties. The preservative is used to maintain the appearance of the [[fruit]] rather than prevent rotting. This can give fruit a distinctive chemical taste. |
| − | Sulfur dioxide is also a good [[Reducing agent|reductant]]. In the presence of water, sulfur dioxide is able to decolorize substances that can be [[Reduction (chemistry)|reduced]] by it; thus making it a useful reducing [[bleach]] for [[paper]]s and delicate materials such as clothes. | + | Sulfur dioxide is also a good [[Reducing agent|reductant]]. In the presence of [[water]], sulfur dioxide is able to decolorize substances that can be [[Reduction (chemistry)|reduced]] by it; thus making it a useful reducing [[bleach]] for [[paper]]s and delicate materials such as clothes. |
This bleaching effect normally does not last very long. [[Oxygen]] in the atmosphere reoxidizes the reduced dyes, restoring the color. | This bleaching effect normally does not last very long. [[Oxygen]] in the atmosphere reoxidizes the reduced dyes, restoring the color. | ||
| Line 156: | Line 156: | ||
Sulfur dioxide is also used to make sulfuric acid, being converted to [[sulfur trioxide]], and then to [[oleum]], which is made into [[sulfuric acid]]. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. This is called the [[contact process]]. | Sulfur dioxide is also used to make sulfuric acid, being converted to [[sulfur trioxide]], and then to [[oleum]], which is made into [[sulfuric acid]]. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. This is called the [[contact process]]. | ||
| − | According to [[Claude Ribbe]] in ''[[The Crime of Napoleon]],'' sulfur dioxide gas was used as an [[gas chamber|execution poison]] by the French emperor to suppress a slave revolt in Haiti early in the | + | According to [[Claude Ribbe]] in ''[[The Crime of Napoleon]],'' sulfur dioxide gas was used as an [[gas chamber|execution poison]] by the French emperor to suppress a slave revolt in Haiti early in the nineteenth century. |
Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors (PSR's) and abolishes the [[Hering-Breuer reflex|Hering-Breuer inflation reflex]]. | Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors (PSR's) and abolishes the [[Hering-Breuer reflex|Hering-Breuer inflation reflex]]. | ||
| Line 164: | Line 164: | ||
Sulfur dioxide is the [[anhydride]] of [[sulfurous acid]], H<sub>2</sub>SO<sub>3</sub>. | Sulfur dioxide is the [[anhydride]] of [[sulfurous acid]], H<sub>2</sub>SO<sub>3</sub>. | ||
| − | Sulfur dioxide is a very important element in | + | Sulfur dioxide is a very important element in [[wine]]making, and is designated as parts per million in wine. It acts as an antibiotic and antioxidant, protecting wine from spoilage [[organism]]s, [[bacteria]], and oxidation, and also helps to keep volatile [[PH|acidity]] at desirable levels. Sulfur dioxide is responsible for the words "contains sulfites" found on wine labels. Wines with SO<sub>2</sub> concentrations below ten ppm do not require "contains sulfites" on the label by US and EU laws. The upper limit of SO<sub>2</sub> allowed in wine is 350ppm in US, in the EU is 160 ppm for red wines and 210 ppm for white and [[rosé|rosé wines]]. In low concentrations SO<sub>2</sub> is mostly undetected in wine, but at over 50ppm, SO<sub>2</sub> becomes evident in the nose and taste of wine. |
| − | SO<sub>2</sub> is also a very important element in winery sanitation. | + | SO<sub>2</sub> is also a very important element in winery sanitation. Wineries and equipment must be kept very clean, and because bleach cannot be used in a winery, a mixture of SO<sub>2</sub>, water, and citric acid is commonly used to clean hoses, tanks, and other equipment to keep it clean and free of bacteria. |
== Emissions == | == Emissions == | ||
| − | According to the [[United States Environmental Protection Agency|U.S. EPA]] (as presented by the ''2002 World Almanac'' or in chart form <ref>[http://www.epa.gov/air/airtrends/sulfur.html National Trends in Sulfur Dioxide Levels] | + | According to the [[United States Environmental Protection Agency|U.S. EPA]] (as presented by the ''2002 World Almanac'' or in chart form<ref>[http://www.epa.gov/air/airtrends/sulfur.html National Trends in Sulfur Dioxide Levels] [[United States Environmental Protection Agency]]. Retrieved December 20, 2007.</ref>), the following amount of sulfur dioxide was released in the [[U.S.]] per year, measured in thousands of [[short tons]]: |
{| border=1 | {| border=1 | ||
|*'''1999''' | |*'''1999''' | ||
| Line 193: | Line 193: | ||
|} | |} | ||
| − | Due largely to the [[United States|US]] EPA’s [[Acid Rain Program]], the U.S. has witnessed a 33 percent decrease in emissions between 1983 and 2002. | + | Due largely to the [[United States|US]] EPA’s [[Acid Rain Program]], the U.S. has witnessed a 33 percent decrease in emissions between 1983 and 2002. This improvement resulted from [[flue gas desulfurization]], a technology that enables SO<sub>2</sub> to be chemically bound in [[power plant]]s burning sulfur-containing [[coal]] or [[Petroleum|oil]]. In particular, [[calcium oxide|calcium oxide (lime)]] reacts with sulfur dioxide to form [[calcium sulfite]]: |
| − | :CaO + SO<sub>2</sub> → | + | :CaO + SO<sub>2</sub> → CaSO<sub>3</sub> |
| − | Aerobic oxidation <!--right?—> converts this CaSO<sub>3</sub> into CaSO<sub>4</sub>, [[gypsum]]. | + | Aerobic oxidation <!--right?—> converts this CaSO<sub>3</sub> into CaSO<sub>4</sub>, [[gypsum]]. Most gypsum sold in Europe comes from flue gas desulfurization. |
| − | New fuel additive catalysts, such as [[ferox (fuel additive)|ferox]], are being used in gasoline and diesel engines in order to lower the emission of sulfur oxide gases into the atmosphere. | + | New fuel additive catalysts, such as [[ferox (fuel additive)|ferox]], are being used in gasoline and diesel engines in order to lower the emission of sulfur oxide gases into the atmosphere. This is also done by forcing the sulfur into stable mineral salts and mixed mineral sulfates as opposed to sulfuric acid and sulfur oxides. |
| − | As of 2006, [[China]] is the world's largest sulfur dioxide polluter, with 2005 emissions estimated to be 25.49 million tons. This amount represents a 27 | + | As of 2006, [[China]] is the world's largest sulfur dioxide polluter, with 2005 emissions estimated to be 25.49 million tons. This amount represents a 27 percent increase since 2000, and is roughly comparable with U.S. emissions in 1980.<ref>[http://upi.com/NewsTrack/view.php?StoryID=20060922-010840-7049r China has its worst spell of acid rain] [[United Press International]]. Retrieved December 20, 2007.</ref> |
[[Al-Mishraq]], an Iraqi sulfur plant, was the site of a 2004 disaster resulting in the release of massive amounts of sulfur dioxide into the atmosphere. | [[Al-Mishraq]], an Iraqi sulfur plant, was the site of a 2004 disaster resulting in the release of massive amounts of sulfur dioxide into the atmosphere. | ||
| Line 226: | Line 226: | ||
* The values are tabulated for 101.3 kPa [[partial pressure]] of SO<sub>2</sub>. [[Solubility]] of gas in a liquid depends on the gas partial pressure according to [[Henry's law]]. | * The values are tabulated for 101.3 kPa [[partial pressure]] of SO<sub>2</sub>. [[Solubility]] of gas in a liquid depends on the gas partial pressure according to [[Henry's law]]. | ||
| − | * The | + | * The solubility is given for "pure water," i.e., water that contains only SO<sub>2</sub> in the amount at equilibrium with the gas phase. This "pure water" is going to be acidic. The solubility of SO<sub>2</sub> in neutral (or alkaline) water is generally going to be higher because of the [[pH]]-dependent speciation of SO<sub>2</sub> in the solution with the production of [[bisulfite]] and some [[sulfite]] ions. |
== See also == | == See also == | ||
| Line 240: | Line 240: | ||
==References== | ==References== | ||
| − | * Chang, Raymond. 2006. ''Chemistry'', 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031 | + | * Chang, Raymond. 2006. ''Chemistry'', 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031 |
| − | + | * Cotton, F. Albert, and Geoffrey Wilkinson. 1980. ''Advanced Inorganic Chemistry'', 4th ed. New York: Wiley. ISBN 0471027758 | |
| − | * Cotton, F. Albert, and Geoffrey Wilkinson. 1980. ''Advanced Inorganic Chemistry'', 4th ed. New York: Wiley. ISBN 0471027758 | + | * Lide, David R. 2006. ''CRC Handbook of Chemistry and Physics''. 87th ed. Boca Raton, FL: CRC Press. ISBN 0849304873 |
| − | |||
| − | * Lide, David R. 2006. ''CRC Handbook of Chemistry and Physics''. 87th ed. Boca Raton, FL: CRC Press. ISBN 0849304873 | ||
| − | |||
* Wells, A. F. 1984. ''Structural Inorganic Chemistry''. 5th ed. Oxford: Oxford University Press. | * Wells, A. F. 1984. ''Structural Inorganic Chemistry''. 5th ed. Oxford: Oxford University Press. | ||
== External links == | == External links == | ||
| + | All Links Retrieved December 20, 2007. | ||
*[http://www.epa.gov/air/airtrends/sulfur.html United States Environmental Protection Agency Sulfur Dioxide page] | *[http://www.epa.gov/air/airtrends/sulfur.html United States Environmental Protection Agency Sulfur Dioxide page] | ||
*[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0074.htm International Chemical Safety Card 0074] | *[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0074.htm International Chemical Safety Card 0074] | ||
| − | |||
*[http://www.cdc.gov/niosh/npg/npgd0575.html NIOSH Pocket Guide to Chemical Hazards] | *[http://www.cdc.gov/niosh/npg/npgd0575.html NIOSH Pocket Guide to Chemical Hazards] | ||
*[http://www.fedupwithfoodadditives.info/factsheets/Factsulphites.htm Food Intolerance Network] - Sulfite factsheet | *[http://www.fedupwithfoodadditives.info/factsheets/Factsulphites.htm Food Intolerance Network] - Sulfite factsheet | ||
Revision as of 22:30, 20 December 2007
| Sulfur dioxide | |
|---|---|
| |
| General | |
| Systematic name | sulfur dioxide |
| Other names | sulphur dioxide sulfur(IV) oxide sulfurous anhydride sulphurous anhydride |
| Molecular formula | SO2 |
| Molar mass | 64.054 g mol−1 |
| Appearance | colorless gas |
| CAS number | [7446-09-5] |
| EINECS number | 231-195-2 |
| Properties | |
| Density and phase | 2.551 g/L, gas |
| Solubility in water | 9.4 g/100 mL (25 °C) |
| Melting point | −72.4 °C (200.75 K) |
| Boiling point | −10 °C (263 K) |
| Critical Point | 157.2°C at 7.87 MPa |
| Acidity (pKa) | 1.81 |
| Structure | |
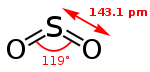
| Molecular shape | Bent 120
[[1] |
| Dipole moment | 1.63 D |
| Thermodynamic data | |
| Standard enthalpy of formation ΔfH°gas |
−296.84 kJ mol−1 |
| Standard molar entropy S°gas |
248.21 J K−1 mol−1 |
| Safety data | |
| EU classification | Toxic |
| R-phrases | R23, R34 |
| S-phrases | S1/2, S9, S26 S36/37/39, S45 |
| NFPA 704 | |
| PEL-TWA (OSHA) | 5 ppm (13 mg m−3) |
| IDLH (NIOSH) | 100 ppm |
| Flash point | non-flammable |
| RTECS number | WS4550000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other cations | Selenium dioxide Tellurium dioxide |
| Related compounds | Sulfur trioxide Sulfuric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula SO2. This important gas is the main product from the combustion of sulfur compounds and is of significant environmental concern. SO2 is often described as the "smell of burning sulfur" but is not responsible for the smell of rotten eggs.
SO2 is produced by volcanoes and in various industrial processes. Since coal and petroleum contain various amounts of sulfur compounds, their combustion generates sulfur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain.[1]
Preparation
Sulfur dioxide can be prepared by burning sulfur in air. This reaction, in which sulfur combines with oxygen in the air, may be written as follows:
- S8(s) + 8O2(g) → 8SO2(g)
The combustion of hydrogen sulfide and organosulfur compounds proceeds in a similar manner:
- 2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g)
Sulfur dioxide is also produced during the roasting of sulfide ores, such as iron pyrites, sphalerite (zinc blende), and cinnabar (mercury sulfide). These reactions are:
- 4FeS2(s) + 11O2(g) → 2Fe2O3(s) + 8SO2(g)
- 2ZnS(s) + 3O2(g) → 2ZnO(s) + 2SO2(g)
- HgS(s) + O2(g) → Hg(g) + SO2(g)
When anhydrous calcium sulfate (CaSO4) is heated with coke and sand in the manufacture of cement, CaSiO3, sulfur dioxide is a by-product.
- 2CaSO4(s) + 2SiO2(s) + C(s) → 2CaSiO3(s) + 2SO2(g) + CO2(g)
The action of hot concentrated sulfuric acid on copper turnings will produce sulfur dioxide:
- Cu(s) + 2H2SO4(aq) → CuSO4(aq) + SO2(g) + 2H2O(l)
Structure and bonding
SO2 is a bent molecule with C2v symmetry point group.
In terms of electron-counting formalisms, the sulfur atom has an oxidation state of +4, a formal charge of zero, and is surrounded by five electron pairs. From the perspective of molecular orbital theory, most of these electron pairs are non-bonding in character, as is typical for hypervalent molecules.
One conventional covalent bond is present between each oxygen and the central sulfur atom, with two further electrons delocalised between the oxygens and the sulfur atom.
Uses
Sulfur dioxide is sometimes used as a preservative (E number: E220[2]) in alcoholic drinks,[3] or dried apricots and other dried fruits due to its antimicrobial properties. The preservative is used to maintain the appearance of the fruit rather than prevent rotting. This can give fruit a distinctive chemical taste.
Sulfur dioxide is also a good reductant. In the presence of water, sulfur dioxide is able to decolorize substances that can be reduced by it; thus making it a useful reducing bleach for papers and delicate materials such as clothes.
This bleaching effect normally does not last very long. Oxygen in the atmosphere reoxidizes the reduced dyes, restoring the color.
Sulfur dioxide is also used to make sulfuric acid, being converted to sulfur trioxide, and then to oleum, which is made into sulfuric acid. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. This is called the contact process.
According to Claude Ribbe in The Crime of Napoleon, sulfur dioxide gas was used as an execution poison by the French emperor to suppress a slave revolt in Haiti early in the nineteenth century.
Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors (PSR's) and abolishes the Hering-Breuer inflation reflex.
Prior to the development of freons, sulfur dioxide was used as a refrigerant in home refrigerators.
Sulfur dioxide is the anhydride of sulfurous acid, H2SO3.
Sulfur dioxide is a very important element in winemaking, and is designated as parts per million in wine. It acts as an antibiotic and antioxidant, protecting wine from spoilage organisms, bacteria, and oxidation, and also helps to keep volatile acidity at desirable levels. Sulfur dioxide is responsible for the words "contains sulfites" found on wine labels. Wines with SO2 concentrations below ten ppm do not require "contains sulfites" on the label by US and EU laws. The upper limit of SO2 allowed in wine is 350ppm in US, in the EU is 160 ppm for red wines and 210 ppm for white and rosé wines. In low concentrations SO2 is mostly undetected in wine, but at over 50ppm, SO2 becomes evident in the nose and taste of wine.
SO2 is also a very important element in winery sanitation. Wineries and equipment must be kept very clean, and because bleach cannot be used in a winery, a mixture of SO2, water, and citric acid is commonly used to clean hoses, tanks, and other equipment to keep it clean and free of bacteria.
Emissions
According to the U.S. EPA (as presented by the 2002 World Almanac or in chart form[4]), the following amount of sulfur dioxide was released in the U.S. per year, measured in thousands of short tons:
| *1999 | 18,867 |
| *1998 | 19,491 |
| *1997 | 19,363 |
| *1996 | 18,859 |
| *1990 | 23,678 |
| *1980 | 25,905 |
| *1970 | 31,161 |
Due largely to the US EPA’s Acid Rain Program, the U.S. has witnessed a 33 percent decrease in emissions between 1983 and 2002. This improvement resulted from flue gas desulfurization, a technology that enables SO2 to be chemically bound in power plants burning sulfur-containing coal or oil. In particular, calcium oxide (lime) reacts with sulfur dioxide to form calcium sulfite:
- CaO + SO2 → CaSO3
Aerobic oxidation converts this CaSO3 into CaSO4, gypsum. Most gypsum sold in Europe comes from flue gas desulfurization.
New fuel additive catalysts, such as ferox, are being used in gasoline and diesel engines in order to lower the emission of sulfur oxide gases into the atmosphere. This is also done by forcing the sulfur into stable mineral salts and mixed mineral sulfates as opposed to sulfuric acid and sulfur oxides.
As of 2006, China is the world's largest sulfur dioxide polluter, with 2005 emissions estimated to be 25.49 million tons. This amount represents a 27 percent increase since 2000, and is roughly comparable with U.S. emissions in 1980.[5]
Al-Mishraq, an Iraqi sulfur plant, was the site of a 2004 disaster resulting in the release of massive amounts of sulfur dioxide into the atmosphere.
Temperature dependence of aqueous solubility
| 22 g/100ml (0 °C) | 15 g/100ml (10 °C) |
| 11 g/100ml (20 °C) | 9.4 g/100 ml (25 °C) |
| 8 g/100ml (30 °C) | 6.5 g/100ml (40 °C) |
| 5 g/100ml (50 °C) | 4 g/100ml (60 °C) |
| 3.5 g/100ml (70 °C) | 3.4 g/100ml (80 °C) |
| 3.5 g/100ml (90 °C) | 3.7 g/100ml (100 °C) |
- The values are tabulated for 101.3 kPa partial pressure of SO2. Solubility of gas in a liquid depends on the gas partial pressure according to Henry's law.
- The solubility is given for "pure water," i.e., water that contains only SO2 in the amount at equilibrium with the gas phase. This "pure water" is going to be acidic. The solubility of SO2 in neutral (or alkaline) water is generally going to be higher because of the pH-dependent speciation of SO2 in the solution with the production of bisulfite and some sulfite ions.
See also
- Oxide
- Sulfur
- Sulfur trioxide
- Sulfuric acid
Notes
- ↑ Dr. Mike Thompson, Winchester College, UK. Online Link Retrieved December 20, 2007.
- ↑ Current EU approved additives and their E Numbers The Food Standards Agency website. Retrieved December 20, 2007.
- ↑ "All wines contain sulphur dioxide in various forms, collectively known as sulphites. Even in completely unsulphured wine it is present at concentrations of up to ten milligrams per litre." Sulphites in wine MoreThanOrganic.com. Retrieved December 20, 2007.
- ↑ National Trends in Sulfur Dioxide Levels United States Environmental Protection Agency. Retrieved December 20, 2007.
- ↑ China has its worst spell of acid rain United Press International. Retrieved December 20, 2007.
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. 2006. Chemistry, 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031
- Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry, 4th ed. New York: Wiley. ISBN 0471027758
- Lide, David R. 2006. CRC Handbook of Chemistry and Physics. 87th ed. Boca Raton, FL: CRC Press. ISBN 0849304873
- Wells, A. F. 1984. Structural Inorganic Chemistry. 5th ed. Oxford: Oxford University Press.
External links
All Links Retrieved December 20, 2007.
- United States Environmental Protection Agency Sulfur Dioxide page
- International Chemical Safety Card 0074
- NIOSH Pocket Guide to Chemical Hazards
- Food Intolerance Network - Sulfite factsheet
- Sulfur Dioxide, Molecule of the Month
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
