Difference between revisions of "Materials science" - New World Encyclopedia
| Line 49: | Line 49: | ||
== Classes of materials == | == Classes of materials == | ||
| − | Materials science encompasses various classes of materials, | + | Materials science encompasses various classes of materials, some of which overlap. Examples are: |
# [[Ionic bond|Ionic crystals]] (crystals in which the atoms are held together by ionic bonds) | # [[Ionic bond|Ionic crystals]] (crystals in which the atoms are held together by ionic bonds) | ||
# [[Covalent bond|Covalent crystals]] (crystals in which the atoms are held together by covalent bonds) | # [[Covalent bond|Covalent crystals]] (crystals in which the atoms are held together by covalent bonds) | ||
# [[Metal]]s | # [[Metal]]s | ||
# [[Intermetallics]]* | # [[Intermetallics]]* | ||
| − | |||
# [[Polymer]]s | # [[Polymer]]s | ||
# [[Composite material]]*s | # [[Composite material]]*s | ||
# [[Vitreous material]]*s | # [[Vitreous material]]*s | ||
| + | # [[Ceramic]]s and [[refractory|refractories]]: These are high-temperature materials, including reinforced carbon-carbon (RCC), polycrystalline [[silicon carbide]]*, and [[transformation-toughened ceramics]]*. | ||
| + | # [[Biomaterial]]s (materials derived from or used with biological systems) | ||
| + | # [[Electronic Materials|Electronic]]* and [[Magnetic Materials|magnetic]]* materials (materials such as [[semiconductors]] used to create [[integrated circuits]], [[storage media]]*, [[sensors]]*, and other devices) | ||
| + | |||
Each class of materials may involve a separate field of study. | Each class of materials may involve a separate field of study. | ||
| − | == | + | ==Subfields of materials science== |
| − | * [[Nanotechnology]]: As commonly understood, nanotechnology is the creation and study of materials | + | * [[Nanotechnology]]: As commonly understood, nanotechnology is the creation and study of materials that have a width ranging from less than 1 [[nanometer]]* (nm) (10<sup>−9</sup> meter) to 100 nm. These materials are generally engineered on a molecular scale. On a more rigorous level, nanoscience involves the study of materials where the defining properties are present only at the nanoscale. |
| − | * [[Crystallography]] | + | * [[Crystallography]]: This is the study of how atoms in a crystalline solid fill space. It includes the determination of [[crystallographic defect|defects]]* associated with [[crystal]] structures and the relationship between these structures and their physical properties. |
| − | * [[Characterization (materials science)|Materials Characterization]] | + | * [[Characterization (materials science)|Materials Characterization]]: To understand and define the properties of materials, they may be characterized by techniques such as diffraction of [[X rays]], [[electrons]], or [[neutrons]], and various forms of [[spectroscopy]], [[chromatography]], [[thermal analysis]]*, or [[electron microscopy]]. |
| − | * [[Metallurgy]] | + | * [[Metallurgy]]: This involves the study of metals and their alloys, including their extraction, [[microstructure]]*, and processing. |
| − | * | + | * [[Tribology]]: This is the study of the wear of materials due to [[friction]] and other factors. |
| − | + | * [[Surface chemistry|Surface science]]: It involves study of the structures and interactions between solid-gas, solid-liquid, and solid-solid interfaces. | |
| − | * [[Tribology]] | + | * [[Glass science]]: It involves the study of noncrystalline materials, including inorganic glasses, vitreous metals and non-oxide glasses. |
| − | * [[Surface chemistry|Surface science]] | + | Some practitioners consider [[rheology]] a subfield of materials science, because it can cover any material that flows. Modern rheology, however, typically deals with non-Newtonian [[fluid dynamics]]*, so it is often considered a subfield of [[continuum mechanics]]*. |
| − | * [[ | ||
| − | Some practitioners | ||
| − | * | ||
===Topics that form the basis of materials science=== | ===Topics that form the basis of materials science=== | ||
| Line 78: | Line 78: | ||
* [[Thermodynamics]], [[statistical mechanics]], [[chemical kinetics|kinetics]] and [[physical chemistry]], for [[phase (matter)|phase]] stability, transformations (physical and chemical) and diagrams. | * [[Thermodynamics]], [[statistical mechanics]], [[chemical kinetics|kinetics]] and [[physical chemistry]], for [[phase (matter)|phase]] stability, transformations (physical and chemical) and diagrams. | ||
* [[Crystallography]] and [[chemical bonding]], for understanding how atoms in a material are arranged. | * [[Crystallography]] and [[chemical bonding]], for understanding how atoms in a material are arranged. | ||
| − | * [[Mechanics]] | + | * [[Mechanics]] of materials: To understand the mechanical properties of materials and their structural applications. |
* [[Solid-state physics]] and [[quantum mechanics]], for the understanding of the electronic, thermal, magnetic, chemical, structural and optical properties of materials. | * [[Solid-state physics]] and [[quantum mechanics]], for the understanding of the electronic, thermal, magnetic, chemical, structural and optical properties of materials. | ||
* [[Diffraction]] and [[wave mechanics]], for the characterization of materials. | * [[Diffraction]] and [[wave mechanics]], for the characterization of materials. | ||
Revision as of 22:58, 6 November 2006
Materials science is an interdisciplinary field involving the study of different types of materials and the applications of this knowledge to various areas of science and engineering. It includes elements of applied physics and chemistry, as well as chemical, mechanical, civil and electrical engineering. With the recent surge of interest in nanoscience and nanotechnology, materials science has been propelled to the forefront at many academic institutions and research facilities.
Besides its importance for industrial applications, materials science has gradually developed into a field that provides tests for condensed matter or solid state theories. New physics emerges because of the diverse new material properties needed to be explained.
Historical overview
Materials science is one of the oldest forms of applied science and engineering. In the development of human civilization, the defining point of each era has often been described in terms of the human ability to work with a new type of material. Examples are the Stone Age, Bronze Age, and Steel Age.
Before the 1960s, (and in some cases decades after), many materials science departments at academic and research institutions were named metallurgy departments, because the emphasis was on the study of metals and their uses. The field has since broadened to include every class of materials, such as ceramics, polymers, semiconductors, magnetic materials, and biological materials (such as medical implants).
Many important elements of modern materials science have resulted from the space race. In particular, the understanding and engineering of metallic alloys, ceramics, and other materials were useful for the construction of space vehicles, space suits, and so forth, and the new knowledge was found valuable for various consumer and industrial applications as well.
Fundamentals of materials science
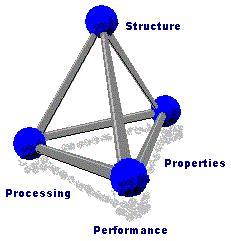
In materials science, the researcher conducts a systematic investigation of each material, in terms of its structure, properties, processing, and performance. The research often leads to new applications of known materials and the creation of new materials with desired properties.
On a fundamental level, this field relates the properties and performance of a material to its atomic-scale structure and the different phases it can go through. The major factors that determine the structure and properties of a material are the nature of its constituent chemical elements and the way in which the material was processed into its final form. These factors, related through the laws of thermodynamics, govern the material’s microstructure, and thus its properties.
The manufacture of a perfect crystal of a material is physically impossible. Instead, materials scientists manipulate the defects in crystalline materials, thereby creating a material with the desired properties. On an atomic scale, the defects in a crystal could mean that atoms of one element may be missing or replaced by atoms of other elements.
Furthermore, not all materials have a regular crystalline structure. Glasses, some ceramics, and many natural materials are amorphous—not possessing any long-range order in their atomic arrangements. These materials are much harder to engineer than crystalline materials. Polymers are a mixed case, with varying degrees of crystallinity, and their study commonly combines elements of chemical and statistical thermodynamics to give thermodynamic (rather than mechanical) descriptions of physical properties.
Materials in Industry
Radical materials advances drive the creation of new products and even new industries. At the same time, stable industries employ materials scientists to make incremental improvements and troubleshoot issues with currently used materials. Industrial applications of materials science include the design of materials and their cost-benefit tradeoffs in industrial production.
Techniques used for processing materials include:
Techniques used for analyzing (characterizing) materials include:
- electron microscopy
- X-ray diffraction
- calorimetry
- nuclear microscopy (HEFIB)
- Rutherford backscattering
- neutron diffraction
The overlap between physics and materials science has led to the offshoot field of materials physics, which is concerned with the physical properties of materials. The approach is generally more macroscopic and applied than in condensed matter physics.
Classes of materials
Materials science encompasses various classes of materials, some of which overlap. Examples are:
- Ionic crystals (crystals in which the atoms are held together by ionic bonds)
- Covalent crystals (crystals in which the atoms are held together by covalent bonds)
- Metals
- Intermetallics
- Polymers
- Composite materials
- Vitreous materials
- Ceramics and refractories: These are high-temperature materials, including reinforced carbon-carbon (RCC), polycrystalline silicon carbide, and transformation-toughened ceramics.
- Biomaterials (materials derived from or used with biological systems)
- Electronic and magnetic materials (materials such as semiconductors used to create integrated circuits, storage media, sensors, and other devices)
Each class of materials may involve a separate field of study.
Subfields of materials science
- Nanotechnology: As commonly understood, nanotechnology is the creation and study of materials that have a width ranging from less than 1 nanometer (nm) (10−9 meter) to 100 nm. These materials are generally engineered on a molecular scale. On a more rigorous level, nanoscience involves the study of materials where the defining properties are present only at the nanoscale.
- Crystallography: This is the study of how atoms in a crystalline solid fill space. It includes the determination of defects associated with crystal structures and the relationship between these structures and their physical properties.
- Materials Characterization: To understand and define the properties of materials, they may be characterized by techniques such as diffraction of X rays, electrons, or neutrons, and various forms of spectroscopy, chromatography, thermal analysis, or electron microscopy.
- Metallurgy: This involves the study of metals and their alloys, including their extraction, microstructure, and processing.
- Tribology: This is the study of the wear of materials due to friction and other factors.
- Surface science: It involves study of the structures and interactions between solid-gas, solid-liquid, and solid-solid interfaces.
- Glass science: It involves the study of noncrystalline materials, including inorganic glasses, vitreous metals and non-oxide glasses.
Some practitioners consider rheology a subfield of materials science, because it can cover any material that flows. Modern rheology, however, typically deals with non-Newtonian fluid dynamics, so it is often considered a subfield of continuum mechanics.
Topics that form the basis of materials science
- Thermodynamics, statistical mechanics, kinetics and physical chemistry, for phase stability, transformations (physical and chemical) and diagrams.
- Crystallography and chemical bonding, for understanding how atoms in a material are arranged.
- Mechanics of materials: To understand the mechanical properties of materials and their structural applications.
- Solid-state physics and quantum mechanics, for the understanding of the electronic, thermal, magnetic, chemical, structural and optical properties of materials.
- Diffraction and wave mechanics, for the characterization of materials.
- Chemistry and polymer science, for the understanding of plastics, colloids, ceramics, liquid crystals, solid state chemistry, and polymers.
- Biology, for the integration of materials into biological systems.
- Continuum mechanics and statistics, for the study of fluid flows and ensemble systems.
- Mechanics of materials, for the study of the relation between the mechanical behavior of materials and their microstructures.
Some nonacademic materials science research facilities
Government laboratories
- Argonne National Laboratory
- Lawrence Berkeley National Laboratory
- Lawrence Livermore National Laboratory
- Los Alamos National Laboratory
- Max Planck Institute
- Oak Ridge National Laboratory
Corporate facilities
- DuPont
- GE Global Research
- IBM Thomas J. Watson Research Center
Significant journals
- Nature Materials
- Acta Materialia
- JOM
- Advanced Materials
- Computational materials science
- Advanced Functional Materials
- Journal of Materials Chemistry
- Journal of Materials Online - Open Access
- Metallurgical and Materials Transactions
ReferencesISBN links support NWE through referral fees
- Askeland, Donald R. and Pradeep P. Phulé (2005). The Science & Engineering of Materials, 5th edition, Thomson-Engineering. ISBN 0-534-55396-6.
- Gaskell, David R. (1995). Introduction to the Thermodynamics of Materials, 4th edition, Taylor and Francis Publishing. ISBN 1-56032-992-0.
- Eberhart, Mark (2003). Why Things Break : Understanding the World by the Way It Comes Apart. Harmony. ISBN 1-4000-4760-9.
- Gordon, James Edward (1984). The New Science of Strong Materials or Why You Don't Fall Through the Floor, eissue edition, Princeton University Press. ISBN 0-691-02380-8.
See also
- Alloy
- Ceramic
- Crystal
- Glass
- Metal
- Plastic
- Polymer
- Timeline of materials technology
- Bio-based materials
- Liquid crystal
| ||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.