Difference between revisions of "Hydrazine" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
Rosie Tanabe (talk | contribs) |
||
| (9 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Copyedited}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} | ||
{{Chembox new | {{Chembox new | ||
| − | | | + | | Name = Hydrazine |
| − | | | + | | ImageFile = Hydrazine-2D.png |
| − | | | + | | ImageFile1 = Hydrazine-3D-balls.png |
| − | | | + | | ImageFile2 = Hydrazine-3D-vdW.png |
| − | | | + | | IUPACName = Hydrazine |
| − | | | + | | OtherNames = |
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| − | | | + | | CASNo = 302-01-2 |
| − | | | + | | RTECS = MU7175000 |
| − | + | }} | |
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| − | | | + | | Formula = N<sub>2</sub>H<sub>4</sub> |
| − | | | + | | MolarMass = 32.05 g/mol |
| − | | | + | | Appearance = Colourless liquid |
| − | | | + | | Density = 1.01 g/mL (liquid) |
| − | | | + | | Solubility = miscible |
| − | | | + | | MeltingPt = 1 °C (274 K) |
| − | | | + | | BoilingPt = 114 °C (387 K) |
| − | | | + | | Viscosity = 0.9 [[Poise|cP]] at 25°C<ref name="Greenwood 1997">N.N. Greenwood, and A. Earnshaw, ''Chemistry of the Elements'' (Oxford, UK: Butterworth-Heinemann, 1997, ISBN 0750633654).</ref> |
| − | + | }} | |
| Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| − | | | + | | MolShape = pyramidal at N |
| − | | | + | | Dipole = 1.85 [[Debye|D]]<ref name="Greenwood 1997"/> |
| − | + | }} | |
| Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| − | | | + | | MainHazards = Toxic,<br />causes burns |
| − | | | + | | NFPA-H = 3 |
| − | | | + | | NFPA-F = 3 |
| − | | | + | | NFPA-R = 3 |
| − | | | + | | FlashPt = 37.78°C (closed cup) |
| − | | | + | | RPhrases = 45-10-23/24/25-34-43-50/53 |
| − | | | + | | SPhrases = 53-45-60-61 |
| − | | | + | | ExternalMSDS = [http://www.sciencelab.com/xMSDS-Hydrazine-9924279 External MSDS] |
| − | + | }} | |
| Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| − | | | + | | Function = hydrides |
| − | | | + | | OtherFunctn = [[hydrogen peroxide]] |
| − | | | + | | OtherCpds = [[ammonia]]<br />[[monomethylhydrazine]]<br />[[dimethylhydrazine]]<br/>[[phenylhydrazine]] |
| − | + | }} | |
}} | }} | ||
| − | '''Hydrazine''' is | + | '''Hydrazine''' is a [[chemical compound]] with the [[chemical formula|formula]] [[Nitrogen|N]]<sub>2</sub>[[Hydrogen|H]]<sub>4</sub>. It has an [[ammonia]]-like odor, and its liquid range and [[density]] are similar to those of [[water]]. It is widely used in chemical synthetic reactions, and it is a component of some [[rocket fuel]]s. It is, however, very toxic and dangerously unstable, especially when not mixed with water. |
| + | {{toc}} | ||
| + | ==Molecular structure and properties== | ||
| − | + | Hydrazine has a simple molecular structure. Its formula may be written as H<sub>2</sub>N-NH<sub>2</sub>, to indicate that there is a [[covalent bond]] between the two [[nitrogen]] [[atom]]s. Conceptually, this structure would arise by coupling a pair of [[ammonia]] (NH<sub>3</sub>) molecules to form the N-N bond, accompanied by the loss of one hydrogen atom per ammonia molecule. | |
| − | Conceptually, | + | |
| + | Within a hydrazine molecule, each H<sub>2</sub>N-N subunit has a pyramidal structure. The N-N distance is 1.45 angstroms (Å), and the molecule adopts a [[gauche conformation]].<ref> Gary L. Miessler, and Donald A. Tarr, ''Inorganic Chemistry'', 3rd Edition'' (Upper Saddle River, NJ: Pearson Education, 2004, ISBN 0-13-035471-6).</ref> The [[rotational barrier]] is twice that of [[ethane]]. These structural properties resemble those of gaseous [[hydrogen peroxide]], which adopts a "skewed" [[Linear alkane conformation| anticlinal]] conformation, and also experiences a strong rotational barrier. | ||
| − | + | Like ammonia, hydrazine is a chemical [[Base (chemistry)|base]], but it is 15 times weaker than ammonia. It can receive a proton (H<sup>+</sup>) as follows: | |
| − | :N<sub>2</sub>H<sub>4</sub> | + | :N<sub>2</sub>H<sub>4</sub> + H<sup>+</sup> → [N<sub>2</sub>H<sub>5</sub>]<sup>+</sup> (K = 8.5 x 10<sup>-7</sup>) |
| − | (for ammonia K = 1.78 x 10<sup>-5</sup>) | + | (for ammonia, K = 1.78 x 10<sup>-5</sup>) |
| − | + | Protonated hydrazine can combine with a second proton with difficulty:<ref>A.F. Holleman, and E. Wiberg, ''Inorganic Chemistry'' (San Diego, CA: Academic Press, 2001, ISBN 0-12-352651-5).</ref> | |
| − | :[N<sub>2</sub>H<sub>5</sub>]<sup>+</sup> | + | :[N<sub>2</sub>H<sub>5</sub>]<sup>+</sup> + H<sup>+</sup> → [N<sub>2</sub>H<sub>6</sub>]<sup>2+</sup> (K = 8.4 x 10<sup>-16</sup>) |
==Synthesis== | ==Synthesis== | ||
| − | |||
| − | + | Free hydrazine was synthesized for the first time by [[Theodor Curtius]] in 1889, by a circuitous route.<ref>J. Prakt Curtius, 1889, ''Chem'' 39:107-39.</ref> | |
| + | |||
| + | Another synthetic process (called the [[Olin Raschig process]]) was announced in 1907, involving the use of [[sodium hypochlorite]] and [[ammonia]]. This method relies on the reaction of [[chloramine]] with ammonia.<ref>R. Adams, and B.K. Brown, 1941, [http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0309 Hydrazine Sulfate] ''Organic Syntheses, Coll.'' 1:309. Retrieved February 25, 2008.</ref> | ||
| − | In the [[Atofina-PCUK cycle]], hydrazine is produced in several steps from [[acetone]], ammonia, and hydrogen peroxide. | + | In the [[Atofina-PCUK cycle]], hydrazine is produced in several steps from [[acetone]], ammonia, and hydrogen peroxide. Acetone and ammonia first react to give the [[imine]], followed by oxidation with [[hydrogen peroxide]] to the [[oxaziridine]], a three-membered ring containing carbon, oxygen, and nitrogen. That is followed by [[ammonolysis]] to the [[hydrazone]], a process that couples two nitrogen atoms. This hydrazone reacts with one more equivalent of acetone, and the resulting azine is hydrolyzed to give hydrazine, regenerating acetone. Unlike the Raschig process, this process does not produce salt. The PCUK stands for Produits Chimiques Ugine Kuhlmann, a French chemical manufacturer.<ref> Emil Raymond Riegel, "Hydrazine" ''Riegel's Handbook of Industrial Chemistry'' (New York, NY: Van Nostrand Reinhold, ISBN 0442001754).</ref> |
Hydrazine can also be produced via the so-called [[ketazine process|ketazine]] and [[peroxide process]]es. | Hydrazine can also be produced via the so-called [[ketazine process|ketazine]] and [[peroxide process]]es. | ||
| − | In 2001, | + | In 2001, microbiologist Marc Strous of the University of Nijmegen in the [[Netherlands]] discovered that hydrazine is produced from yeast bacteria and the open ocean bacterium anammox ''(Brocadia anammoxidans)''. They are the only organisms known to produce hydrazine naturally.<ref>Brian Handwerk, 2005, [http://news.nationalgeographic.com/news/2005/11/1109_051109_rocketfuel.html Bacteria Eat Human Sewage, Produce Rocket Fuel] National Geographic. Retrieved February 25, 2008.</ref> |
==Hydrazine derivatives== | ==Hydrazine derivatives== | ||
| − | Many substituted hydrazines are known, | + | |
| − | *[[gyromitrin]] and [[agaritine]] are phenylhydrazines found in the commercially produced mushroom species ''[[Agaricus bisporus]]''. | + | Many substituted hydrazines are known, several of which occur naturally. Some examples include: |
| + | *[[gyromitrin]] and [[agaritine]] are phenylhydrazines found in the commercially produced [[mushroom]] [[species]] ''[[Agaricus bisporus]]''. Gyromitrin is metabolized into [[monomethyl hydrazine]]. | ||
*[[iproniazid]], [[hydralazine]] and [[phenelzine]] are hydrazine-containing [[medication]]s. | *[[iproniazid]], [[hydralazine]] and [[phenelzine]] are hydrazine-containing [[medication]]s. | ||
| − | *[[UDMH|1,1-dimethylhydrazine]] and [[1,2-dimethylhydrazine]] are hydrazines where two hydrogen atoms are replaced by [[methyl group]]s. | + | *[[UDMH|1,1-dimethylhydrazine]] and [[1,2-dimethylhydrazine]] are hydrazines where two [[hydrogen]] atoms are replaced by [[methyl group]]s. |
*[[2,4-Dinitrophenylhydrazine|2,4-dinitrophenylhydrazine]] (2,4-DNP) is commonly used to test for [[ketones]] and [[aldehydes]] in [[organic chemistry]]. | *[[2,4-Dinitrophenylhydrazine|2,4-dinitrophenylhydrazine]] (2,4-DNP) is commonly used to test for [[ketones]] and [[aldehydes]] in [[organic chemistry]]. | ||
*[[phenylhydrazine]], C<sub>6</sub>H<sub>5</sub>NHNH<sub>2</sub>, the first hydrazine to be discovered. | *[[phenylhydrazine]], C<sub>6</sub>H<sub>5</sub>NHNH<sub>2</sub>, the first hydrazine to be discovered. | ||
==Uses in chemistry== | ==Uses in chemistry== | ||
| − | Hydrazines are part of many [[organic syntheses]], | + | |
| + | Hydrazines are part of many [[organic syntheses]], many of which are of practical significance in [[pharmaceutical]]s, such as [[antituberculant]]s, as well as in textile [[dye]]s and in photography. | ||
===Hydrazone formation=== | ===Hydrazone formation=== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | In a related reaction 2-cyano[[pyridine]]s react with hydrazine to form amide hydrazides which can be converted using 1,2-diketones into [[triazines]]. | + | Hydrazine undergoes a condensation reaction with [[acetone]] to give the [[azine]]. This azine reacts further with hydrazine to produce the hydrazone:<ref>A.C. Day, and M.C. Whiting, 1988, [http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0010 Acetone Hydrazone] ''Organic Syntheses, Coll.'' 6:10. Retrieved February 25, 2008.</ref> |
| + | :2 (CH<sub>3</sub>)<sub>2</sub>CO + N<sub>2</sub>H<sub>4</sub> → 2 H<sub>2</sub>O + [(CH<sub>3</sub>)<sub>2</sub>C=N]<sub>2</sub> | ||
| + | :[(CH<sub>3</sub>)<sub>2</sub>C=N]<sub>2</sub> + N<sub>2</sub>H<sub>4</sub> → 2 (CH<sub>3</sub>)<sub>2</sub>C=NNH<sub>2</sub> | ||
| + | The acetone azine is an intermediate in the Atofina-PCUK synthesis. Direct [[alkylation]] of hydrazines with [[alkyl halides]] in the presence of base produces alkyl-substituted hydrazines, but the reaction is typically inefficient due to poor control on the level of substitution (same as in ordinary [[amine]]s). The reduction of [[hydrazone]]s to hydrazines present a clean way to produce 1,1-dialkylated hydrazines. | ||
| + | |||
| + | In a related reaction 2-cyano[[pyridine]]s react with hydrazine to form amide hydrazides, which can be converted using 1,2-diketones into [[triazines]]. | ||
===Wolff-Kishner reduction=== | ===Wolff-Kishner reduction=== | ||
| − | Hydrazine is used in the [[Wolff-Kishner reduction]], a reaction that transforms the [[carbonyl]] group of a [[ketone]] or [[aldehyde]] into a [[methylene]] (or [[methyl]]) group via a [[hydrazone]] intermediate. | + | |
| + | Hydrazine is used in the [[Wolff-Kishner reduction]], a reaction that transforms the [[carbonyl]] group of a [[ketone]] or [[aldehyde]] into a [[methylene]] (or [[methyl]]) group via a [[hydrazone]] intermediate. The production of the highly stable [[dinitrogen]] from the hydrazine derivative helps drive the reaction. | ||
===Heterocyclic chemistry=== | ===Heterocyclic chemistry=== | ||
| − | Being bifunctional, with two | + | |
| + | Being bifunctional, with two amine groups, hydrazine is a key building block for the preparation of many heterocyclic compounds via condensation, with a range of difunctional [[electrophiles]]. With [[2,4-pentanedione]], it condenses to give the [[3,5-dimethylpyrazole]].<ref>R.H. Wiley, and P.E. Hexner, 1963, [http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0351 3,5-Dimethylpyrazole] ''Organic Syntheses, Coll.'' 4:351. Retrieved February 25, 2008.</ref> In the [[Einhorn-Brunner reaction]], hydrazines react with imides to give [[triazole]]s. | ||
===Sulfonation=== | ===Sulfonation=== | ||
| − | Being a good nucleophile, N<sub>2</sub>H<sub>4</sub> is susceptible to attack by sulfonyl halides and acyl halides.<ref> | + | |
| + | Being a good nucleophile, N<sub>2</sub>H<sub>4</sub> is susceptible to attack by sulfonyl halides and acyl halides.<ref>L. Friedman, R.L. Litle, and W.R. Reichle, 1973, [http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p1055 ''p''-Toluenesulfonyl Hydrazide] ''Organic Syntheses, Coll''. 5:1055. Retrieved February 25, 2008.</ref> The [[tosyl]]hydrazine also forms hydrazones upon treatment with carbonyls. | ||
===Deprotection of phthalimides=== | ===Deprotection of phthalimides=== | ||
| − | Hydrazine is used to cleave ''N''-alkylated phthalimide derivatives. | + | |
| + | Hydrazine is used to cleave ''N''-alkylated phthalimide derivatives. This scission reaction allows the phthalimide anion to be used as an amine precursor in the [[Gabriel synthesis]].<ref>N.M. Weinshenker, C.M. Shen, and J.Y. Wong, 1988, [http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0951 Polymeric carbodiimide] ''Organic Syntheses, Coll''. 6:951. Retrieved February 25, 2008.</ref> | ||
===Reducing agent=== | ===Reducing agent=== | ||
| − | Hydrazine is a convenient reductant because the by-products are typically nitrogen gas and water. | + | |
| + | Hydrazine is a convenient reductant because the by-products are typically nitrogen gas and water. Thus, it is used as an [[antioxidant]], an oxygen [[scavenger (chemistry)|scavenger]], and a [[corrosion inhibitor]] in water boilers and heating systems. It is also used to reduce metal salts and oxides to the pure metals in [[electroless nickel plating|electroless]] [[nickel]] plating and [[plutonium]] extraction from [[nuclear waste|nuclear reactor waste]]. | ||
===Hydrazinium salts=== | ===Hydrazinium salts=== | ||
| − | Hydrazine is converted to solid salts by treatment with mineral acids. | + | |
| + | Hydrazine is converted to solid salts by treatment with mineral acids. A common salt is hydrazine hydrogen [[sulfate]], [N<sub>2</sub>H<sub>5</sub>]HSO<sub>4</sub>, which probably should be called hydrazinium bisulfate. Hydrazine bisulfate is used as an alternative treatment of cancer-induced [[cachexia]]. The salt of hydrazine and [[hydrazoic acid]] N<sub>5</sub>H<sub>5</sub> was of scientific interest, because of the high nitrogen content and the explosive properties. | ||
==Other industrial uses== | ==Other industrial uses== | ||
| − | Hydrazine is used in many processes | + | |
| + | Hydrazine is used in many processes. Examples include: production of [[spandex]] fibers, as a [[polymerization]] [[catalyst]]; a [[blowing agent]]; in [[fuel cell]]s, [[solder]] [[flux (metallurgy)|fluxes]]; and [[photographic developer]]s, as a [[chain extender]] in [[polyurethane|urethane]] polymerizations, and heat stabilizers. In addition, a semiconductor deposition technique using hydrazine has recently been demonstrated, with possible application to the manufacture of [[thin-film transistor]]s used in [[liquid crystal display]]s. A solution of 70 percent hydrazine and 30 percent water is used to power the EPU ([[emergency power unit]]) on the [[F-16]] fighter plane. The explosive [[Astrolite]] is made by combining hydrazine with [[ammonium nitrate]]. | ||
===Rocket fuel=== | ===Rocket fuel=== | ||
| − | |||
| − | Hydrazine is also used as a low-power [[monopropellant]] for the maneuvering thrusters of spacecraft, and the [[Space Shuttle]]'s Auxiliary Power Units. | + | Hydrazine was first used as a [[rocket fuel]] during [[World War II]] for the [[Messerschmitt Me 163#Me 163 B|Messerschmitt Me 163B]] (the first rocket-powered fighter plane), under the name ''B-Stoff'' (hydrazine [[hydrate]]) and in a mixture with [[methanol]] ([[M-Stoff]]) and [[hydrogen peroxide]] called [[C-Stoff]]. |
| + | |||
| + | Hydrazine is also used as a low-power [[monopropellant]] for the maneuvering thrusters of spacecraft, and the [[Space Shuttle]]'s Auxiliary Power Units. In addition, monopropellant hydrazine-fueled rocket engines are often used in terminal descent of spacecraft. A collection of such engines were used in both [[Viking program|Viking]] landers as well as the [[Phoenix (spacecraft)|Phoenix]] lander launched in August 2007. | ||
| − | In all hydrazine monopropellant engines the hydrazine is passed by a [[catalyst]] such as [[iridium]] metal supported by high-surface-area [[alumina]] ( | + | In all hydrazine monopropellant engines, the hydrazine is passed by a [[catalyst]] such as [[iridium]] metal supported by high-surface-area [[alumina]] (aluminum oxide) or [[carbon nanofiber]]s,<ref name="Vieira">R. Vieira, C. Pham-Huu, N. Keller and M. J. Ledoux, 2002, New carbon nanofiber/graphite felt composite for use as a catalyst support for hydrazine catalytic decomposition, ''Chemical Communications'' 9:954—955.</ref> or more recently [[molybdenum nitride]] on alumina,<ref name="Chen">Xiaowei Chen, et al., 2002, Catalytic Decomposition of Hydrazine over Supported Molybdenum Nitride Catalysts in a Monopropellant Thruster, ''Catalysis Letters.'' 79:21–25.</ref> which causes it to decompose into [[ammonia]], nitrogen gas, and hydrogen gas according to the following reactions: |
#3 N<sub>2</sub>H<sub>4</sub> → 4 NH<sub>3</sub> + N<sub>2</sub> | #3 N<sub>2</sub>H<sub>4</sub> → 4 NH<sub>3</sub> + N<sub>2</sub> | ||
| Line 115: | Line 131: | ||
These reactions are extremely [[exothermic]] (the catalyst chamber can reach 800 °C in a matter of milliseconds<ref name="Vieira" />), and they produce large volumes of hot gas from a small volume of liquid hydrazine,<ref name="Chen" /> making it an efficient thruster propellant. | These reactions are extremely [[exothermic]] (the catalyst chamber can reach 800 °C in a matter of milliseconds<ref name="Vieira" />), and they produce large volumes of hot gas from a small volume of liquid hydrazine,<ref name="Chen" /> making it an efficient thruster propellant. | ||
| − | Other variants of | + | Other variants of hydrazine that are used as rocket fuel are [[monomethylhydrazine]], CH<sub>3</sub>NHNH<sub>2</sub> (also known as MMH) and [[unsymmetrical dimethylhydrazine]], (CH<sub>3</sub>)<sub>2</sub>NNH<sub>2</sub> (also known as UDMH). These are used as two-component rocket fuel, often together with [[dinitrogen tetroxide]], N<sub>2</sub>O<sub>4</sub>. |
| − | == | + | ==Toxicity== |
| − | |||
| − | == | + | Hydrazine is highly toxic and dangerously unstable, especially in the [[anhydrous]] form. Symptoms of acute exposure to high levels of hydrazine may include irritation of the eyes, nose, and throat, dizziness, headache, nausea, [[pulmonary edema]], seizures, and coma in humans. Acute exposure can also damage the [[liver]], [[kidney]]s, and central [[nervous system]] in [[human]]s. The liquid is corrosive and may produce [[dermatitis]] from skin contact in humans and [[animal]]s. Effects to the lungs, liver, spleen, and thyroid have been reported in animals chronically exposed to hydrazine via inhalation. Increased incidences of lung, nasal cavity, and liver tumors have been observed in rodents exposed to hydrazine. |
| − | + | ||
| + | ==See also== | ||
| + | |||
| + | * [[Ammonia]] | ||
| + | * [[Hydrogen peroxide]] | ||
| + | * [[Nitrogen]] | ||
| + | |||
| + | == Notes == | ||
<references /> | <references /> | ||
| − | |||
| − | == | + | ==References== |
| − | * | + | * Parker, Phillip M. 2006. ''The 2007 Import and Export Market for Organic Derivatives of Hydrazine or Hydroxylamine in United Kingdom''. San Diego, CA: ICON Group International, Inc. ISBN 0546028152 |
| + | * Schmidt, Eckart Walter. 2001. ''Hydrazine and its Derivatives : Preparation, Properties, Applications''. New York, NY: Wiley-Interscience. ISBN 0471415537 | ||
| + | * Toth, Bela. 2000. ''Hydrazines and Cancer: A Guidebook on the Carcinogenic Activities of Hydrazines, Related Chemicals, and Hydrazine Containing Natural Products''. Amsterdam, NL: Harwood Academic Publishers. ISBN 9057026317 | ||
==External links== | ==External links== | ||
| − | * [http://www.princeton.edu/~orggroup/supergroup_pdf/rmatunasAGM5hydrazine.pdf The Late Show with Rob! Tonight’s Special Guest: Hydrazine (PDF)] | + | All links retrieved January 22, 2018. |
| + | * Matunas, Robert. [http://www.princeton.edu/~orggroup/supergroup_pdf/rmatunasAGM5hydrazine.pdf The Late Show with Rob! Tonight’s Special Guest: Hydrazine (PDF)] | ||
| − | [[Category: | + | [[Category:Physical sciences]] |
| − | [[Category: | + | [[Category:Chemistry]] |
| − | [[Category: | + | [[Category:Inorganic chemistry]] |
| − | [[Category: | + | [[Category:Environmental science]] |
| − | [[Category: | + | [[Category:Energy technology]] |
| − | |||
| − | |||
| − | + | {{credit|175501595}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 19:54, 22 January 2018
| Hydrazine | |
|---|---|
| |
| |
| |
| IUPAC name | Hydrazine |
| Identifiers | |
| CAS number | [] |
| RTECS number | MU7175000 |
| Properties | |
| Molecular formula | N2H4 |
| Molar mass | 32.05 g/mol |
| Appearance | Colourless liquid |
| Density | 1.01 g/mL (liquid) |
| Melting point |
1 °C (274 K) |
| Boiling point |
114 °C (387 K) |
| Solubility in water | miscible |
| Viscosity | 0.9 cP at 25°C[1] |
| Structure | |
| Molecular shape | pyramidal at N |
| Dipole moment | 1.85 D[1] |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Toxic, causes burns |
| NFPA 704 |
|
| R-phrases | 45-10-23/24/25-34-43-50/53 |
| S-phrases | 53-45-60-61 |
| Flash point | 37.78°C (closed cup) |
| Related Compounds | |
| Related hydrides | hydrogen peroxide |
| Related compounds | ammonia monomethylhydrazine dimethylhydrazine phenylhydrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
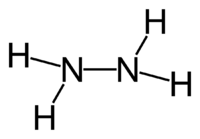
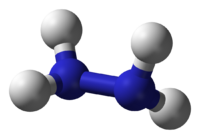
Hydrazine is a chemical compound with the formula N2H4. It has an ammonia-like odor, and its liquid range and density are similar to those of water. It is widely used in chemical synthetic reactions, and it is a component of some rocket fuels. It is, however, very toxic and dangerously unstable, especially when not mixed with water.
Molecular structure and properties
Hydrazine has a simple molecular structure. Its formula may be written as H2N-NH2, to indicate that there is a covalent bond between the two nitrogen atoms. Conceptually, this structure would arise by coupling a pair of ammonia (NH3) molecules to form the N-N bond, accompanied by the loss of one hydrogen atom per ammonia molecule.
Within a hydrazine molecule, each H2N-N subunit has a pyramidal structure. The N-N distance is 1.45 angstroms (Å), and the molecule adopts a gauche conformation.[2] The rotational barrier is twice that of ethane. These structural properties resemble those of gaseous hydrogen peroxide, which adopts a "skewed" anticlinal conformation, and also experiences a strong rotational barrier.
Like ammonia, hydrazine is a chemical base, but it is 15 times weaker than ammonia. It can receive a proton (H+) as follows:
- N2H4 + H+ → [N2H5]+ (K = 8.5 x 10-7)
(for ammonia, K = 1.78 x 10-5) Protonated hydrazine can combine with a second proton with difficulty:[3]
- [N2H5]+ + H+ → [N2H6]2+ (K = 8.4 x 10-16)
Synthesis
Free hydrazine was synthesized for the first time by Theodor Curtius in 1889, by a circuitous route.[4]
Another synthetic process (called the Olin Raschig process) was announced in 1907, involving the use of sodium hypochlorite and ammonia. This method relies on the reaction of chloramine with ammonia.[5]
In the Atofina-PCUK cycle, hydrazine is produced in several steps from acetone, ammonia, and hydrogen peroxide. Acetone and ammonia first react to give the imine, followed by oxidation with hydrogen peroxide to the oxaziridine, a three-membered ring containing carbon, oxygen, and nitrogen. That is followed by ammonolysis to the hydrazone, a process that couples two nitrogen atoms. This hydrazone reacts with one more equivalent of acetone, and the resulting azine is hydrolyzed to give hydrazine, regenerating acetone. Unlike the Raschig process, this process does not produce salt. The PCUK stands for Produits Chimiques Ugine Kuhlmann, a French chemical manufacturer.[6]
Hydrazine can also be produced via the so-called ketazine and peroxide processes.
In 2001, microbiologist Marc Strous of the University of Nijmegen in the Netherlands discovered that hydrazine is produced from yeast bacteria and the open ocean bacterium anammox (Brocadia anammoxidans). They are the only organisms known to produce hydrazine naturally.[7]
Hydrazine derivatives
Many substituted hydrazines are known, several of which occur naturally. Some examples include:
- gyromitrin and agaritine are phenylhydrazines found in the commercially produced mushroom species Agaricus bisporus. Gyromitrin is metabolized into monomethyl hydrazine.
- iproniazid, hydralazine and phenelzine are hydrazine-containing medications.
- 1,1-dimethylhydrazine and 1,2-dimethylhydrazine are hydrazines where two hydrogen atoms are replaced by methyl groups.
- 2,4-dinitrophenylhydrazine (2,4-DNP) is commonly used to test for ketones and aldehydes in organic chemistry.
- phenylhydrazine, C6H5NHNH2, the first hydrazine to be discovered.
Uses in chemistry
Hydrazines are part of many organic syntheses, many of which are of practical significance in pharmaceuticals, such as antituberculants, as well as in textile dyes and in photography.
Hydrazone formation
Hydrazine undergoes a condensation reaction with acetone to give the azine. This azine reacts further with hydrazine to produce the hydrazone:[8]
- 2 (CH3)2CO + N2H4 → 2 H2O + [(CH3)2C=N]2
- [(CH3)2C=N]2 + N2H4 → 2 (CH3)2C=NNH2
The acetone azine is an intermediate in the Atofina-PCUK synthesis. Direct alkylation of hydrazines with alkyl halides in the presence of base produces alkyl-substituted hydrazines, but the reaction is typically inefficient due to poor control on the level of substitution (same as in ordinary amines). The reduction of hydrazones to hydrazines present a clean way to produce 1,1-dialkylated hydrazines.
In a related reaction 2-cyanopyridines react with hydrazine to form amide hydrazides, which can be converted using 1,2-diketones into triazines.
Wolff-Kishner reduction
Hydrazine is used in the Wolff-Kishner reduction, a reaction that transforms the carbonyl group of a ketone or aldehyde into a methylene (or methyl) group via a hydrazone intermediate. The production of the highly stable dinitrogen from the hydrazine derivative helps drive the reaction.
Heterocyclic chemistry
Being bifunctional, with two amine groups, hydrazine is a key building block for the preparation of many heterocyclic compounds via condensation, with a range of difunctional electrophiles. With 2,4-pentanedione, it condenses to give the 3,5-dimethylpyrazole.[9] In the Einhorn-Brunner reaction, hydrazines react with imides to give triazoles.
Sulfonation
Being a good nucleophile, N2H4 is susceptible to attack by sulfonyl halides and acyl halides.[10] The tosylhydrazine also forms hydrazones upon treatment with carbonyls.
Deprotection of phthalimides
Hydrazine is used to cleave N-alkylated phthalimide derivatives. This scission reaction allows the phthalimide anion to be used as an amine precursor in the Gabriel synthesis.[11]
Reducing agent
Hydrazine is a convenient reductant because the by-products are typically nitrogen gas and water. Thus, it is used as an antioxidant, an oxygen scavenger, and a corrosion inhibitor in water boilers and heating systems. It is also used to reduce metal salts and oxides to the pure metals in electroless nickel plating and plutonium extraction from nuclear reactor waste.
Hydrazinium salts
Hydrazine is converted to solid salts by treatment with mineral acids. A common salt is hydrazine hydrogen sulfate, [N2H5]HSO4, which probably should be called hydrazinium bisulfate. Hydrazine bisulfate is used as an alternative treatment of cancer-induced cachexia. The salt of hydrazine and hydrazoic acid N5H5 was of scientific interest, because of the high nitrogen content and the explosive properties.
Other industrial uses
Hydrazine is used in many processes. Examples include: production of spandex fibers, as a polymerization catalyst; a blowing agent; in fuel cells, solder fluxes; and photographic developers, as a chain extender in urethane polymerizations, and heat stabilizers. In addition, a semiconductor deposition technique using hydrazine has recently been demonstrated, with possible application to the manufacture of thin-film transistors used in liquid crystal displays. A solution of 70 percent hydrazine and 30 percent water is used to power the EPU (emergency power unit) on the F-16 fighter plane. The explosive Astrolite is made by combining hydrazine with ammonium nitrate.
Rocket fuel
Hydrazine was first used as a rocket fuel during World War II for the Messerschmitt Me 163B (the first rocket-powered fighter plane), under the name B-Stoff (hydrazine hydrate) and in a mixture with methanol (M-Stoff) and hydrogen peroxide called C-Stoff.
Hydrazine is also used as a low-power monopropellant for the maneuvering thrusters of spacecraft, and the Space Shuttle's Auxiliary Power Units. In addition, monopropellant hydrazine-fueled rocket engines are often used in terminal descent of spacecraft. A collection of such engines were used in both Viking landers as well as the Phoenix lander launched in August 2007.
In all hydrazine monopropellant engines, the hydrazine is passed by a catalyst such as iridium metal supported by high-surface-area alumina (aluminum oxide) or carbon nanofibers,[12] or more recently molybdenum nitride on alumina,[13] which causes it to decompose into ammonia, nitrogen gas, and hydrogen gas according to the following reactions:
- 3 N2H4 → 4 NH3 + N2
- N2H4 → N2 + 2 H2
- 4 NH3 + N2H4 → 3 N2 + 8 H2
These reactions are extremely exothermic (the catalyst chamber can reach 800 °C in a matter of milliseconds[12]), and they produce large volumes of hot gas from a small volume of liquid hydrazine,[13] making it an efficient thruster propellant.
Other variants of hydrazine that are used as rocket fuel are monomethylhydrazine, CH3NHNH2 (also known as MMH) and unsymmetrical dimethylhydrazine, (CH3)2NNH2 (also known as UDMH). These are used as two-component rocket fuel, often together with dinitrogen tetroxide, N2O4.
Toxicity
Hydrazine is highly toxic and dangerously unstable, especially in the anhydrous form. Symptoms of acute exposure to high levels of hydrazine may include irritation of the eyes, nose, and throat, dizziness, headache, nausea, pulmonary edema, seizures, and coma in humans. Acute exposure can also damage the liver, kidneys, and central nervous system in humans. The liquid is corrosive and may produce dermatitis from skin contact in humans and animals. Effects to the lungs, liver, spleen, and thyroid have been reported in animals chronically exposed to hydrazine via inhalation. Increased incidences of lung, nasal cavity, and liver tumors have been observed in rodents exposed to hydrazine.
See also
Notes
- ↑ 1.0 1.1 N.N. Greenwood, and A. Earnshaw, Chemistry of the Elements (Oxford, UK: Butterworth-Heinemann, 1997, ISBN 0750633654).
- ↑ Gary L. Miessler, and Donald A. Tarr, Inorganic Chemistry, 3rd Edition (Upper Saddle River, NJ: Pearson Education, 2004, ISBN 0-13-035471-6).
- ↑ A.F. Holleman, and E. Wiberg, Inorganic Chemistry (San Diego, CA: Academic Press, 2001, ISBN 0-12-352651-5).
- ↑ J. Prakt Curtius, 1889, Chem 39:107-39.
- ↑ R. Adams, and B.K. Brown, 1941, Hydrazine Sulfate Organic Syntheses, Coll. 1:309. Retrieved February 25, 2008.
- ↑ Emil Raymond Riegel, "Hydrazine" Riegel's Handbook of Industrial Chemistry (New York, NY: Van Nostrand Reinhold, ISBN 0442001754).
- ↑ Brian Handwerk, 2005, Bacteria Eat Human Sewage, Produce Rocket Fuel National Geographic. Retrieved February 25, 2008.
- ↑ A.C. Day, and M.C. Whiting, 1988, Acetone Hydrazone Organic Syntheses, Coll. 6:10. Retrieved February 25, 2008.
- ↑ R.H. Wiley, and P.E. Hexner, 1963, 3,5-Dimethylpyrazole Organic Syntheses, Coll. 4:351. Retrieved February 25, 2008.
- ↑ L. Friedman, R.L. Litle, and W.R. Reichle, 1973, p-Toluenesulfonyl Hydrazide Organic Syntheses, Coll. 5:1055. Retrieved February 25, 2008.
- ↑ N.M. Weinshenker, C.M. Shen, and J.Y. Wong, 1988, Polymeric carbodiimide Organic Syntheses, Coll. 6:951. Retrieved February 25, 2008.
- ↑ 12.0 12.1 R. Vieira, C. Pham-Huu, N. Keller and M. J. Ledoux, 2002, New carbon nanofiber/graphite felt composite for use as a catalyst support for hydrazine catalytic decomposition, Chemical Communications 9:954—955.
- ↑ 13.0 13.1 Xiaowei Chen, et al., 2002, Catalytic Decomposition of Hydrazine over Supported Molybdenum Nitride Catalysts in a Monopropellant Thruster, Catalysis Letters. 79:21–25.
ReferencesISBN links support NWE through referral fees
- Parker, Phillip M. 2006. The 2007 Import and Export Market for Organic Derivatives of Hydrazine or Hydroxylamine in United Kingdom. San Diego, CA: ICON Group International, Inc. ISBN 0546028152
- Schmidt, Eckart Walter. 2001. Hydrazine and its Derivatives : Preparation, Properties, Applications. New York, NY: Wiley-Interscience. ISBN 0471415537
- Toth, Bela. 2000. Hydrazines and Cancer: A Guidebook on the Carcinogenic Activities of Hydrazines, Related Chemicals, and Hydrazine Containing Natural Products. Amsterdam, NL: Harwood Academic Publishers. ISBN 9057026317
External links
All links retrieved January 22, 2018.
- Matunas, Robert. The Late Show with Rob! Tonight’s Special Guest: Hydrazine (PDF)
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
