Difference between revisions of "Crystal" - New World Encyclopedia
(→External links: added credit for "Crystal structure") |
|||
| Line 1: | Line 1: | ||
{{dablink|For other senses of this word, see [[crystal (disambiguation)]].}} | {{dablink|For other senses of this word, see [[crystal (disambiguation)]].}} | ||
| − | [[image:Quartz Crystal.jpg|thumb|250px| | + | [[image:Quartz Crystal.jpg|thumb|250px|A quartz crystal.]] |
In [[chemistry]] and [[mineralogy]], a '''crystal''' is a [[solid]] in which the constituent [[atom]]s, [[molecule]]s, or [[Ion (physics)|ion]]s are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is called '''[[crystallography]]*'''. | In [[chemistry]] and [[mineralogy]], a '''crystal''' is a [[solid]] in which the constituent [[atom]]s, [[molecule]]s, or [[Ion (physics)|ion]]s are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is called '''[[crystallography]]*'''. | ||
| Line 14: | Line 14: | ||
[[Image:Insulincrystals.jpg|thumb|250px|Crystals of insulin.]] | [[Image:Insulincrystals.jpg|thumb|250px|Crystals of insulin.]] | ||
| − | Crystalline structures occur in all classes of materials, with all types of [[chemical bond]]s. Almost all [[metal]]s exist in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. [[Ionic bond | Ionically bonded]] | + | Crystalline structures occur in all classes of materials, with all types of [[chemical bond]]s. Almost all [[metal]]s exist in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. [[Ionic bond|Ionically bonded]] crystals can form upon solidification of salts, either from a molten fluid or when it condenses from a solution. [[Covalent]]ly bonded crystals are also common, notable examples being diamond, silica, and graphite. [[Polymer]] materials generally form crystalline regions, but the lengths of the molecules usually prevents complete crystallization. Weak [[Van der Waals force]]s can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in [[graphite]]. |
Most crystalline materials have a variety of [[crystallographic defect]]*s. The types and structures of these defects can have a profound effect on the properties of the materials. | Most crystalline materials have a variety of [[crystallographic defect]]*s. The types and structures of these defects can have a profound effect on the properties of the materials. | ||
| Line 151: | Line 151: | ||
Incommensurate crystals have period-varying translational symmetry. The period between nodes of symmetry is constant in most crystals. The distance between nodes in an incommensurate crystal is dependent on the number of nodes between it and the base node. | Incommensurate crystals have period-varying translational symmetry. The period between nodes of symmetry is constant in most crystals. The distance between nodes in an incommensurate crystal is dependent on the number of nodes between it and the base node. | ||
| + | |||
| + | == Crystal habit == | ||
| + | In [[mineralogy]], shape and size give rise to descriptive terms applied to the typical appearance, or '''habit''' of [[crystal]]s. | ||
| + | |||
| + | [[image:pyrite sun.jpg|right|thumb|300px|Pyrite sun (or dollar) in laminated shale matrix. Between tightly spaced layers of shale, the aggregate was forced to grow in a laterally compressed, radiating manner. Under normal conditions, pyrite would form cubes or pyritohedrons.]] | ||
| + | |||
| + | The many terms used by mineralogists to describe crystal habits are useful in communicating what specimens of a particular mineral often look like. Recognising numerous habits helps a mineralogist to identify a large number of minerals. Some habits are distinctive of certain minerals, although most minerals exhibit many differing habits which are influenced by certain factors. Crystal habit may mislead the inexperienced as a mineral's [[crystal structure|crystal system]] can be hidden or disguised. | ||
| + | |||
| + | Factors influencing a crystal's habit include: a combination of two or more [[crystal form|form]]s; trace impurities present during growth; [[crystal twinning]] and growth conditions (i.e., heat, pressure, space). Minerals belonging to the same crystal system do not necessarily exhibit the same habit. Some habits of a mineral are unique to its variety and locality: For example, while most [[sapphire]]s form elongate barrel-shaped crystals, those found in [[Montana]] form stout ''tabular'' crystals. Ordinarily, the latter habit is seen only in [[ruby]]. Sapphire and ruby are both varieties of the same mineral; [[corundum]]. | ||
| + | |||
| + | Some minerals may replace other existing minerals while preserving the original's habit: this process is called [[pseudomorphous replacement]]. A classic example is [[tiger's eye]] [[quartz]], [[crocidolite]] [[asbestos]] replaced by [[silica]]. While quartz typically forms ''euhedral'' (well-formed), ''prismatic'' (elongate, prism-like) crystals, in tiger's eye the original ''fibrous'' habit of crocidolite is preserved. | ||
| + | |||
| + | ===List of crystal habits=== | ||
| + | |||
| + | {| border="1" cellpadding="2" | ||
| + | |'''Habit:''' | ||
| + | |'''Description:''' | ||
| + | |'''Example:''' | ||
| + | |- | ||
| + | |Acicular | ||
| + | |Needle-like, slender and/or tapered | ||
| + | |[[Rutile]]* in quartz | ||
| + | |- | ||
| + | |Amygdaloidal | ||
| + | |Almond-shaped | ||
| + | |[[Heulandite]]* | ||
| + | |- | ||
| + | |Anhedral | ||
| + | |Poorly formed, external crystal faces not developed | ||
| + | |[[Olivine]]* | ||
| + | |- | ||
| + | |Bladed | ||
| + | |Blade-like, slender and flattened | ||
| + | |[[Kyanite]]* | ||
| + | |- | ||
| + | |Botryoidal or globular | ||
| + | |Grape-like, hemispherical masses | ||
| + | |[[Smithsonite]]* | ||
| + | |- | ||
| + | |Columnar | ||
| + | |Similar to fibrous: Long, slender prisms often with parallel growth | ||
| + | |[[Calcite]]* | ||
| + | |- | ||
| + | |Coxcomb | ||
| + | |Aggregated flaky or tabular crystals closely spaced. | ||
| + | |[[Barite]]* | ||
| + | |- | ||
| + | |Dendritic or arborescent | ||
| + | |Tree-like, branching in one or more direction from central point | ||
| + | |[[Magnesite]]* in [[opal]] | ||
| + | |- | ||
| + | |Dodecahedral | ||
| + | |Dodecahedron, 12-sided | ||
| + | |[[Garnet]] | ||
| + | |- | ||
| + | |Drusy or encrustation | ||
| + | |Aggregate of minute crystals coating a surface | ||
| + | |[[Uvarovite]]* | ||
| + | |- | ||
| + | |Enantiomorphic | ||
| + | |Mirror-image habit and optical characteristics; right- and left-handed crystals | ||
| + | |[[Quartz]] | ||
| + | |- | ||
| + | |Equant, stout, stubby or blocky | ||
| + | |Squashed, pinnacoids dominant over prisms | ||
| + | |[[Zircon]]* | ||
| + | |- | ||
| + | |Euhedral | ||
| + | |Well-formed, external crystal faces developed | ||
| + | |[[Spinel]]* | ||
| + | |- | ||
| + | |Fibrous or columnar | ||
| + | |Extremely slender prisms | ||
| + | |[[Tremolite]]* | ||
| + | |- | ||
| + | |Filiform or capillary | ||
| + | |Hair-like or thread-like, extremely fine | ||
| + | |[[Natrolite]]* | ||
| + | |- | ||
| + | |Foliated or micaceous | ||
| + | |Layered structure, parting into thin sheets | ||
| + | |[[Mica]] | ||
| + | |- | ||
| + | |Granular | ||
| + | |Aggregates of anhedral crystals in matrix | ||
| + | |[[Scheelite]]* | ||
| + | |- | ||
| + | |Hemimorphic | ||
| + | |Doubly terminated crystal with two differently shaped ends. | ||
| + | |[[Hemimorphite]]* | ||
| + | |- | ||
| + | |Mamillary | ||
| + | |Breast-like: intersecting large rounded contours | ||
| + | |[[Malachite]]* | ||
| + | |- | ||
| + | |Massive or compact | ||
| + | |Shapeless, no distinctive external crystal shape | ||
| + | |[[Serpentine]]* | ||
| + | |- | ||
| + | |Nodular or tuberose | ||
| + | |Deposit of roughly spherical form with irregular protuberances | ||
| + | |[[Geode]]s | ||
| + | |- | ||
| + | |Octahedral | ||
| + | |Octahedron, eight-sided (two pyramids base to base) | ||
| + | |[[Diamond]] | ||
| + | |- | ||
| + | |Plumose | ||
| + | |Fine, feather-like scales | ||
| + | |[[Mottramite]]* | ||
| + | |- | ||
| + | |Prismatic | ||
| + | |Elongate, prism-like: all crystal faces parallel to [[c-axis]]* | ||
| + | |[[Tourmaline]]* | ||
| + | |- | ||
| + | |Pseudo-hexagonal | ||
| + | |Ostensibly hexagonal due to cyclic twinning | ||
| + | |[[Aragonite]]* | ||
| + | |- | ||
| + | |Pseudomorphous | ||
| + | |Occurring in the shape of another mineral through pseudomorphous replacement | ||
| + | |[[Tiger's eye]]* | ||
| + | |- | ||
| + | |Radiating or divergent | ||
| + | |Radiating outward from a central point | ||
| + | |[[Pyrite]]* suns | ||
| + | |- | ||
| + | |Reniform or colloform | ||
| + | |Similar to mamillary: intersecting kidney-shaped masses | ||
| + | |[[Hematite]]* | ||
| + | |- | ||
| + | |Reticulated | ||
| + | |Acicular crystals forming net-like intergrowths | ||
| + | |[[Cerussite]]* | ||
| + | |- | ||
| + | |Rosette | ||
| + | |Platy, radiating rose-like aggregate | ||
| + | |[[Gypsum]]* | ||
| + | |- | ||
| + | |Sphenoid | ||
| + | |Wedge-shaped | ||
| + | |[[Sphene]]* | ||
| + | |- | ||
| + | |Stalactitic | ||
| + | |Forming as stalactites or stalagmites; cylindrical or cone-shaped | ||
| + | |[[Rhodochrosite]]* | ||
| + | |- | ||
| + | |Stellate | ||
| + | |Star-like, radiating | ||
| + | |[[Pyrophyllite]]* | ||
| + | |- | ||
| + | |Striated/striations | ||
| + | |Surface growth lines parallel or perpendicular to c-axis | ||
| + | |[[Chrysoberyl]]* | ||
| + | |- | ||
| + | |Subhedral | ||
| + | |External crystal faces only partially developed | ||
| + | | | ||
| + | |- | ||
| + | |Tabular or lamellar | ||
| + | |Flat, tablet-shaped, prominent pinnacoid | ||
| + | |[[Ruby]] | ||
| + | |- | ||
| + | |Wheat sheaf | ||
| + | |Aggregates resembling hand-reaped wheat sheaves | ||
| + | |[[Zeolite]]*s | ||
| + | |} | ||
==Historical and mythical uses of crystals== | ==Historical and mythical uses of crystals== | ||
Revision as of 00:11, 18 October 2006
- For other senses of this word, see crystal (disambiguation).
In chemistry and mineralogy, a crystal is a solid in which the constituent atoms, molecules, or ions are packed in a regularly ordered, repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is called crystallography.
Generally, crystals form when they undergo a process of solidification.<crystallization?> Under ideal conditions, the result may be a single crystal, with all the atoms of the solid fitting into the same crystal structure. Generally, however, many crystals form simultaneously during solidification, leading to a polycrystalline solid. For example, most metals encountered in everyday life are polycrystals. In addition, crystals are often symmetrically intergrown to form crystal twins.
Which crystal structure will be formed from a fluid depends on the chemistry of the fluid, the conditions under which it is being solidified, and the ambient pressure. The process of forming a crystalline structure is often referred to as crystallization.
While the cooling process usually results in the generation of a crystalline material, under certain conditions, the fluid may be frozen in a noncrystalline state. In most cases, this involves cooling the fluid so rapidly that atoms cannot travel to their lattice sites before they lose mobility. A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material. It is also often referred to as an amorphous solid, although there are distinct differences between solids and glasses: most notably, the process of forming a glass does not release the latent heat of fusion. For this reason, many scientists consider glassy materials to be viscous liquids rather than solids, although this is a controversial topic.
Crystalline structures occur in all classes of materials, with all types of chemical bonds. Almost all metals exist in a polycrystalline state; amorphous or single-crystal metals must be produced synthetically, often with great difficulty. Ionically bonded crystals can form upon solidification of salts, either from a molten fluid or when it condenses from a solution. Covalently bonded crystals are also common, notable examples being diamond, silica, and graphite. Polymer materials generally form crystalline regions, but the lengths of the molecules usually prevents complete crystallization. Weak Van der Waals forces can also play a role in a crystal structure; for example, this type of bonding loosely holds together the hexagonal-patterned sheets in graphite.
Most crystalline materials have a variety of crystallographic defects. The types and structures of these defects can have a profound effect on the properties of the materials.
While the term "crystal" has a precise meaning within materials science and solid-state physics, colloquially "crystal" refers to solid objects that exhibit well-defined geometric shapes, often pleasing in appearance. In this sense of the word, many types of crystals are found in nature. The shape of these crystals depends on the types of molecular bonds between the atoms to determine the structure, as well as on the conditions under which they formed. Snowflakes, diamonds, and common salt are common examples of crystals.
Some crystalline materials may exhibit special electrical properties, such as the ferroelectric effect or the piezoelectric effect. Additionally, light passing through a crystal is often bent in different directions, producing an array of colors; crystal optics is the study of these effects. In periodic dielectric structures a range of unique optical properties can be expected as described in photonic crystals.<?>
Crystallization
Crystallization is the (natural or artificial) process of formation of solid crystals from a homogeneous solution. Crystallization is also a chemical solid-liquid separation technique.
The crystallization process consists of two major events, nucleation and crystal growth.
Nucleation is the step where the solute molecules dispersed in the solvent start to gather to create clusters in the nanometer scale (elevating solute concentration in a small region) as to become stable under the current operating conditions. These stable clusters constitute the nuclei. However when the clusters are not stable, they redissolve. Therefore, the clusters need to reach a critical size in order to become stable nuclei. Such critical size is dictated by the operating conditions (temperature, supersaturation, irregularities, etc.). It is at the stage of nucleation that the atoms arrange in a defined and periodic manner that defines the crystal structure — note that "crystal structure" is a special term that refers to the internal arrangement of the atoms, but NOT the physical external macroscopic properties of the crystal, size and shape.
The crystal growth is the subsequent growth of the nuclei that succeed in achieving the critical cluster size. Subsequently, nucleation and growth continue to occur simultaneously while the supersaturation exists. Supersaturation is the driving force of the crystallization, hence the rate of nucleation and growth is driven by the existing supersaturation in the solution. Depending upon the conditions, either nucleation or growth may be predominant over the other, and as a result, crystals with different sizes and shapes are obtained (Control of crystal size and shape constitutes one of the main challenges in industrial manufacturing, such as for pharmaceuticals). Once the supersaturation is exhausted, the solid-liquid system reaches the equilibrium and the crystallization is completed, unless the operating conditions are modified from equilibrium as to supersaturate the solution again.
Crystallization in nature
There are many examples of crystallization in nature. Examples include the formation of:
- mineral crystals (including gemstones);
- stalactites and stalagmites;
- snowflakes.
Artificial methods
For crystallization to occur the solution must be supersaturated. This means that the solution has to contain more solute entities (molecules or ions) dissolved than it would contain under the equilibrium (saturated solution). This can be achieved by various methods, with 1) solution cooling, 2) addition of a second solvent to reduce the solubility of the solute (technique known as anti-solvent or drown-out), 3) chemical reaction and 4) change in pH being the most common methods used in industrial practice. Other methods, such as solvent evaporation, can also be used.
Crystal structure
In mineralogy and crystallography, a crystal structure is a unique arrangement of atoms in a crystal. A crystal structure is composed of a unit cell, a set of atoms arranged in a particular way; which is periodically repeated in three dimensions on a lattice. The spacing between unit cells in various directions is called its lattice parameters. The symmetry properties of the crystal are embodied in its space group. A crystal's structure and symmetry play a role in determining many of its properties, such as cleavage, electronic band structure, and optical properties.
Unit cell
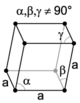
The crystal structure of a material is often discussed in terms of its unit cell. The unit cell is a spatial arrangement of atoms which is tiled in three-dimensional space to describe the crystal. The unit cell is given by its lattice parameters, the length of the cell edges and the angles between them, while the positions of the atoms inside the unit cell are described by the set of atomic positions measured from a lattice point.
For each crystal structure there is a conventional unit cell, which is the smallest unit that has the full symmetry of the crystal (see below). However, the conventional unit cell is not always the smallest possible choice. A primitive unit cell of a particular crystal structure is the smallest possible unit cell one can construct such that, when tiled, it completely fills space. This primitive unit cell does not, however, display all the symmetries inherent in the crystal. A Wigner-Seitz cell is a particular kind of primitive cell which has the same symmetry as the lattice.
Classification of crystals by symmetry
The defining property of a crystal is its inherent symmetry, by which we mean that under certain operations the crystal remains unchanged. For example, rotating the crystal 180 degrees about a certain axis may result in an atomic configuration which is identical to the original configuration. The crystal is then said to have a two-fold rotational symmetry about this axis. In addition to rotational symmetries like this, a crystal may have symmetries in the form of mirror planes and translational symmetries, and also the so-called compound symmetries which are a combination of translation and rotation/mirror symmetries. A full classification of a crystal is achieved when all of these inherent symmetries of the crystal are identified.
Crystal systems
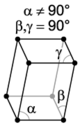
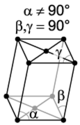
The crystal systems are a grouping of crystal structures according to the axial system used to describe their lattice. Each crystal system consists of a set of three axes in a particular geometrical arrangement. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube, that is, the three axes are mutually perpendicular and of equal length. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic. Some crystallographers consider the hexagonal crystal system not to be its own crystal system, but instead a part of the trigonal crystal system. The crystal system and Bravais lattice of a crystal describe the (purely) translational symmetry of the crystal.
The Bravais lattices
| Crystal system | Lattices | |||
| triclinic | 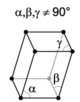
| |||
| monoclinic | simple | base-centered | ||
|
| |||
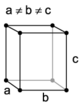
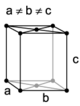
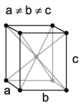
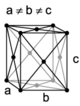
| orthorhombic | simple | base-centered | body-centered | face-centered |
|
|
|
| |
| hexagonal | 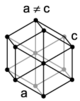
| |||
| rhombohedral (trigonal) |
| |||
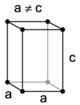
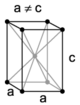
| tetragonal | simple | body-centered | ||
|
| |||
| cubic (isometric) |
simple | body-centered | face-centered | |
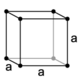
|
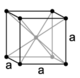
|
Cubic, face-centered | ||
When the crystal systems are combined with the various possible lattice centerings, we arrive at the Bravais lattices. They describe the geometric arrangement of the lattice points, and thereby the translational symmetry of the crystal. In three dimensions, there are 14 unique Bravais lattices which are distinct from one another in the translational symmetry they contain. All crystalline materials recognized until now (not including quasicrystals) fit in one of these arrangements. The fourteen three-dimensional lattices, classified by crystal system, are shown to the right. The Bravais lattices are sometimes referred to as space lattices.
The crystal structure consists of the same group of atoms, the basis, positioned around each and every lattice point. This group of atoms therefore repeats indefinitely in three dimensions according to the arrangement of one of the 14 Bravais lattices. The characteristic rotation and mirror symmetries of the group of atoms, or unit cell, is described by its crystallographic point group.
Point and space groups
The crystallographic point group or crystal class is the set of non-translational symmetry operations that leave the appearance of the crystal structure unchanged. These symmetry operations can include mirror planes, which reflect the structure across a central plane, rotation axes, which rotate the structure a specified number of degrees, and a center of symmetry or inversion point which inverts the structure through a central point. There are 32 possible crystal classes. Each one can be classified into one of the seven crystal systems.
The space group of the crystal structure is composed of the translational symmetry operations in addition to the operations of the point group. These include pure translations which move a point along a vector, screw axes, which rotate a point around an axis while translating parallel to the axis, and glide planes, which reflect a point through a plane while translating it parallel to the plane. There are 230 distinct space groups.
Physical properties
Defects in crystals
Real crystals feature defects or irregularities in the ideal arrangements described above and it is these defects that critically determine many of the electrical and mechanical properties of real materials. In particular dislocations in the crystal lattice allow shear at much lower stress than that needed for a perfect crystal structure.
Crystal symmetry and physical properties
Twenty of the 32 crystal classes are so-called piezoelectric, and crystals belonging to one of these classes (point groups) display piezoelectricity. All 20 piezoelectric classes lack a center of symmetry. Any material develops a dielectric polarization when an electric field is applied, but a substance which has such a natural charge separation even in the absence of a field is called a polar material. Whether or not a material is polar is determined solely by its crystal structure. Only 10 of the 32 point groups are polar. All polar crystals are pyroelectric, so the 10 polar crystal classes are sometimes referred to as the pyroelectric classes.
There are a few crystal structures, notably the perovskite structure, which exhibit ferroelectric behaviour. This is analogous to ferromagnetism, in that, in the absence of an electric field during production, the ferroelectric crystal does not exhibit a polarisation. Upon the application of an electric field of sufficient magnitude, the crystal becomes permanently polarised. This polarisation can be reversed by a sufficiently large counter-charge, in the same way that a ferromagnet can be reversed. However, it is important to note that, although they are called ferroelectrics, the effect is due to the crystal structure, not the presence of a ferrous metal.
Incommensurate crystals have period-varying translational symmetry. The period between nodes of symmetry is constant in most crystals. The distance between nodes in an incommensurate crystal is dependent on the number of nodes between it and the base node.
Crystal habit
In mineralogy, shape and size give rise to descriptive terms applied to the typical appearance, or habit of crystals.
The many terms used by mineralogists to describe crystal habits are useful in communicating what specimens of a particular mineral often look like. Recognising numerous habits helps a mineralogist to identify a large number of minerals. Some habits are distinctive of certain minerals, although most minerals exhibit many differing habits which are influenced by certain factors. Crystal habit may mislead the inexperienced as a mineral's crystal system can be hidden or disguised.
Factors influencing a crystal's habit include: a combination of two or more forms; trace impurities present during growth; crystal twinning and growth conditions (i.e., heat, pressure, space). Minerals belonging to the same crystal system do not necessarily exhibit the same habit. Some habits of a mineral are unique to its variety and locality: For example, while most sapphires form elongate barrel-shaped crystals, those found in Montana form stout tabular crystals. Ordinarily, the latter habit is seen only in ruby. Sapphire and ruby are both varieties of the same mineral; corundum.
Some minerals may replace other existing minerals while preserving the original's habit: this process is called pseudomorphous replacement. A classic example is tiger's eye quartz, crocidolite asbestos replaced by silica. While quartz typically forms euhedral (well-formed), prismatic (elongate, prism-like) crystals, in tiger's eye the original fibrous habit of crocidolite is preserved.
List of crystal habits
| Habit: | Description: | Example: |
| Acicular | Needle-like, slender and/or tapered | Rutile in quartz |
| Amygdaloidal | Almond-shaped | Heulandite |
| Anhedral | Poorly formed, external crystal faces not developed | Olivine |
| Bladed | Blade-like, slender and flattened | Kyanite |
| Botryoidal or globular | Grape-like, hemispherical masses | Smithsonite |
| Columnar | Similar to fibrous: Long, slender prisms often with parallel growth | Calcite |
| Coxcomb | Aggregated flaky or tabular crystals closely spaced. | Barite |
| Dendritic or arborescent | Tree-like, branching in one or more direction from central point | Magnesite in opal |
| Dodecahedral | Dodecahedron, 12-sided | Garnet |
| Drusy or encrustation | Aggregate of minute crystals coating a surface | Uvarovite |
| Enantiomorphic | Mirror-image habit and optical characteristics; right- and left-handed crystals | Quartz |
| Equant, stout, stubby or blocky | Squashed, pinnacoids dominant over prisms | Zircon |
| Euhedral | Well-formed, external crystal faces developed | Spinel |
| Fibrous or columnar | Extremely slender prisms | Tremolite |
| Filiform or capillary | Hair-like or thread-like, extremely fine | Natrolite |
| Foliated or micaceous | Layered structure, parting into thin sheets | Mica |
| Granular | Aggregates of anhedral crystals in matrix | Scheelite |
| Hemimorphic | Doubly terminated crystal with two differently shaped ends. | Hemimorphite |
| Mamillary | Breast-like: intersecting large rounded contours | Malachite |
| Massive or compact | Shapeless, no distinctive external crystal shape | Serpentine |
| Nodular or tuberose | Deposit of roughly spherical form with irregular protuberances | Geodes |
| Octahedral | Octahedron, eight-sided (two pyramids base to base) | Diamond |
| Plumose | Fine, feather-like scales | Mottramite |
| Prismatic | Elongate, prism-like: all crystal faces parallel to c-axis | Tourmaline |
| Pseudo-hexagonal | Ostensibly hexagonal due to cyclic twinning | Aragonite |
| Pseudomorphous | Occurring in the shape of another mineral through pseudomorphous replacement | Tiger's eye |
| Radiating or divergent | Radiating outward from a central point | Pyrite suns |
| Reniform or colloform | Similar to mamillary: intersecting kidney-shaped masses | Hematite |
| Reticulated | Acicular crystals forming net-like intergrowths | Cerussite |
| Rosette | Platy, radiating rose-like aggregate | Gypsum |
| Sphenoid | Wedge-shaped | Sphene |
| Stalactitic | Forming as stalactites or stalagmites; cylindrical or cone-shaped | Rhodochrosite |
| Stellate | Star-like, radiating | Pyrophyllite |
| Striated/striations | Surface growth lines parallel or perpendicular to c-axis | Chrysoberyl |
| Subhedral | External crystal faces only partially developed | |
| Tabular or lamellar | Flat, tablet-shaped, prominent pinnacoid | Ruby |
| Wheat sheaf | Aggregates resembling hand-reaped wheat sheaves | Zeolites |
Historical and mythical uses of crystals
According to Rebbenu Bachya, the word "Achlmah" in the verse Exodus 28:19 means "Crystal" and was the stone on the Ephod representing the tribe of Gad.
Crystals also figure or figured prominently as healing tools in a number of mythologies.
See also
- Atomic packing factor
- Crystal habit
- Crystal structure
- Crystallite
- Crystallization
- Liquid crystal
- Quasicrystal
- Seed crystal
- Single crystal
- Crystal ball
- Crystal oscillator
- Crystal radio
External links
- Chemistry of Crystals
- Introduction to Crystallography and Mineral Crystal Systems
- Crystallographic Teaching Pamphlets
- Crystal Lattice Structures
- A virtual museum about the crystal
- The Giant Crystal Project - documenting the largest crystals and crystal aggregates known to exist
- Industrial Crystallization
- The first record of dumb luck protein crystallization
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.