Difference between revisions of "Chelation" - New World Encyclopedia
m (Protected "Chelation": Copyedited [edit=sysop:move=sysop]) |
|||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Images OK}}{{Approved}}{{Copyedited}} | ||
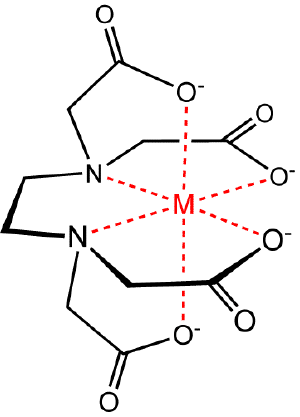
[[Image:Medta.png|thumb|right|Metal-[[EDTA]] chelate.]] | [[Image:Medta.png|thumb|right|Metal-[[EDTA]] chelate.]] | ||
| − | '''Chelation''' | + | '''Chelation''' is the [[binding (molecular)|binding]] or [[complex (chemistry)|complexation]] of a bidentate or multidentate [[ligand]] to a substrate. The ligand, which is often an [[organic compound]], is called a chelant, chelator, chelating agent, or [[sequestration|sequestering agent]]. The substrate is usually a [[metal]] [[ion (physics)|ion]]. The complex formed between the ligand and the substrate is called a '''chelate complex'''. The term ''chelation'' is reserved for complexes in which the metal ion is bound to two or more atoms of the chelating agent. Common chelators include [[citric acid]], [[EDTA]], and [[phosphonate]]s. |
| + | |||
| + | In nature, various [[protein]]s, [[polysaccharide]]s, and [[nucleic acid]]s are good chelators of many metal ions. In addition, metal chelates are important for the mobilization of [[metals]] in the [[soil]], and the uptake of metals by [[plants]] and [[microorganism]]s. | ||
| + | |||
| + | Chelation is useful for various practical applications. For example, chelators are used in [[chemical analysis]], as [[water softener]]s, as ingredients in [[shampoos]] and food [[preservative]]s, and in water treatment programs. In medicine, chelating agents may be used to detoxify a person from [[poison]]ous metals by converting the metals to chemically inert forms. Some advocate the use of chelation as a treatment for [[autism]]. [[Tetracycline]] [[antibiotic]]s are chelators of [[Calcium|Ca]]<sup>2+</sup> and [[Magnesium|Mg]]<sup>2+</sup> ions. EDTA is used in [[endodontic therapy|root canal treatment]], and [[gadolinium]](III) chelates are used as [[contrast medium|contrast agent]]s in [[MRI|MRI scan]]s. Although chelating agents can be beneficial, some can be dangerous under certain circumstances. | ||
==History and etymology== | ==History and etymology== | ||
| − | Chelation is from [[Greek language|Greek]] χηλή, ''chelè'' | + | Chelation is from [[Greek language|Greek]] χηλή, ''chelè,'' meaning "claw". The term ''chelate'' was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the [[lobster]] or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce [[heterocyclic]] rings."<ref>Gilbert T. Morgan and Harry D. K. Drew. CLXII.—Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. [[J. Chem. Soc., Trans.]] 117 (1920): 1456. doi 10.1039/CT9201701456 </ref> |
| − | == The | + | == The Chelate Effect == |
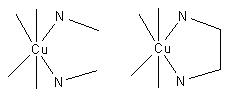
[[image:Cu chelate.png|thumb|Cu<sup>2+</sup> complexes with methylamine (left) end ethylene diamine (right)]] | [[image:Cu chelate.png|thumb|Cu<sup>2+</sup> complexes with methylamine (left) end ethylene diamine (right)]] | ||
Consider the two equilibria, in aqueous solution, between the [[copper]](II) ion, Cu<sup>2+</sup> and [[ethylenediamine]] (en) on the one hand and [[methylamine]], MeNH<sub>2</sub> on the other. | Consider the two equilibria, in aqueous solution, between the [[copper]](II) ion, Cu<sup>2+</sup> and [[ethylenediamine]] (en) on the one hand and [[methylamine]], MeNH<sub>2</sub> on the other. | ||
:Cu<sup>2+</sup> + en {{eqm}} [Cu(en)]<sup>2+</sup> (1) | :Cu<sup>2+</sup> + en {{eqm}} [Cu(en)]<sup>2+</sup> (1) | ||
:Cu<sup>2+</sup> + 2 MeNH<sub>2</sub> {{eqm}} [Cu(MeNH<sub>2</sub>)<sub>2</sub>]<sup>2+</sup> (2) | :Cu<sup>2+</sup> + 2 MeNH<sub>2</sub> {{eqm}} [Cu(MeNH<sub>2</sub>)<sub>2</sub>]<sup>2+</sup> (2) | ||
| − | In (1) the [[bidentate]] ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two [[monodentate]] methylamine ligands of approximately the same donor power, meaning that the [[enthalpy]] of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the [[EDTA]] complex, which has six chelate rings, is | + | In (1) the [[bidentate]] ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two [[monodentate]] methylamine ligands of approximately the same donor power, meaning that the [[enthalpy]] of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal [[copper]] concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the [[EDTA]] complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate [[nitrogen]] donor ligands and four monodentate carboxylate ligands. Thus, the [[phenomenon]] of the chelate effect is a firmly established [[empirical]] fact. |
The [[equilibrium thermodynamics|thermodynamic]] approach to explaining the chelate effect considers the [[equilibrium constant]] for the reaction: the larger the equilibrium constant, the higher the concentration of the complex. | The [[equilibrium thermodynamics|thermodynamic]] approach to explaining the chelate effect considers the [[equilibrium constant]] for the reaction: the larger the equilibrium constant, the higher the concentration of the complex. | ||
| Line 19: | Line 24: | ||
An equilibrium constant, ''K'', is related to the standard [[Gibbs free energy]], Δ''G''<sup>[[Image:StrikeO.png]]</sup> by | An equilibrium constant, ''K'', is related to the standard [[Gibbs free energy]], Δ''G''<sup>[[Image:StrikeO.png]]</sup> by | ||
| − | :Δ[[G]]<sup>[[Image:StrikeO.png]]</sup> = −RT ln ''K'' = Δ''H''<sup> | + | :Δ[[G]]<sup>[[Image:StrikeO.png]]</sup> = −RT ln ''K'' = Δ''H''<sup>Image:StrikeO.png</sup> − TΔ''S''<sup>Image:StrikeO.png</sup> |
| − | where R is the [[gas constant]] and T is the temperature in [[Kelvin]]. Δ''H''<sup>[[Image:StrikeO.png]]</sup> is the standard [[ enthalpy]] change of the reaction and Δ''S''<sup> | + | where R is the [[gas constant]] and T is the temperature in [[Kelvin]]. Δ''H''<sup>[[Image:StrikeO.png]]</sup> is the standard [[ enthalpy]] change of the reaction and Δ''S''<sup>Image:StrikeO.png</sup> is the standard [[Entropy (statistical thermodynamics)|entropy]] change. It has already been posited that the enthalpy term should be approximately the same for the two reactions. Therefore the difference between the two stability constants is due to the entropy term. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less [[Entropy (order and disorder)|entropy of disorder]] is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.<ref name=GE>N.N. Greenwood, & A. Earnshaw. 1997. ''Chemistry of the Elements,'' 2nd Ed. (Oxford, UK: Butterworth-Heinemann. ISBN 0750633654), 910</ref> |
:{| class="wikitable" | :{| class="wikitable" | ||
| − | ! Equilibrium !! log β !! ΔG<sup>[[Image:StrikeO.png]]</sup>!! Δ''H''<sup> | + | ! Equilibrium !! log β !! ΔG<sup>[[Image:StrikeO.png]]</sup>!! Δ''H''<sup>Image:StrikeO.png</sup> /kJ mol<sup>−1</sup>!! −''T''Δ''S''<sup>Image:StrikeO.png</sup> /kJ mol<sup>−1</sup> |
|- | |- | ||
| Cd<sup>2+</sup> + 4 MeNH<sub>2</sub> {{eqm}} Cd(MeNH<sub>2</sub>)<sub>4</sub><sup>2+</sup> | | Cd<sup>2+</sup> + 4 MeNH<sub>2</sub> {{eqm}} Cd(MeNH<sub>2</sub>)<sub>4</sub><sup>2+</sup> | ||
| Line 30: | Line 35: | ||
||10.62|| -60.67 || -56.48||-4.19 | ||10.62|| -60.67 || -56.48||-4.19 | ||
|} | |} | ||
| − | These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is | + | These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favorable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy. |
| − | Other explanations, | + | Other explanations, including that of Schwarzenbach,<ref>G. Schwarzenbach. 1952. Der Chelateffekt. ''Helv. Chim. Acta'' 35:2344–2359. doi 10.1002/hlca.19520350721</ref> are discussed in Greenwood and Earnshaw, 910 ''(loc.cit)''. |
| − | ==Chelation in | + | ==Chelation in Nature== |
| − | + | Many biochemicals exhibit the ability to dissolve certain metal [[cation]]s. For example, [[protein]]s, [[polysaccharide]]s, and [[nucleic acid]]s are excellent polydentate ligands for many metal ions. [[Histidine]], [[malate]], and [[phytochelatin]] are typical chelators used by plants.<ref>U. Krämer, J.D. Cotter-Howells, J M Charnock, A H J M Baker, and J.A.C. Smith. 1996. Free histidine as a metal chelator in plants that accumulate nickel. ''Nature'' 379:635–638. doi 10.1038/379635a0.</ref><ref>Jurandir Vieira Magalhaes, 2006. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1502523 Aluminum tolerance genes are conserved between monocots and dicots]. ''Proc. Natl. Acad. Sci. USA'' 103 (26): 9749-9750. doi=10.1073/pnas.0603957103. pmid=16785425. Retrieved December 24, 2008.</ref><ref>Suk-Bong Ha, Aaron P. Smith, Ross Howden, Wendy M. Dietrich, Sarah Bugg, Matthew J. O'Connell, Peter B. Goldsbrough, and Christopher S. Cobbett. 1999. [http://www.plantcell.org/cgi/content/full/11/6/1153?ck=nck Phytochelatin synthase genes from arabidopsis and the yeast ''Schizosaccharomyces pombe'']. ''Plant Cell''. 11:1153–1164. doi=10.1105/tpc.11.6.1153. pmid=10368185. Retrieved December 24, 2008.</ref> | |
===In biochemistry and microbiology=== | ===In biochemistry and microbiology=== | ||
| − | Virtually all metalloenzymes feature | + | Virtually all metalloenzymes feature [[metal]]s that are chelated, usually to peptides or cofactors and prosthetic groups.<ref>S.J. Lippard and J.M. Berg. 1994. ''Principles of Bioinorganic Chemistry.'' (Mill Valley, CA: University Science Books. ISBN 0935702733.)</ref> Such chelating agents include the [[porphyrin]] rings in [[hemoglobin]] and [[chlorophyll]]. Many microbial species produce water-soluble pigments that serve as chelating agents, termed [[siderophores]]. For example, species of ''Pseudomonas'' are known to secrete [[pycocyanin]] and [[pyoverdin]] that bind iron. [[Enterobactin]], produced by [[E. coli]], is the strongest chelating agent known. |
===In geology=== | ===In geology=== | ||
| − | In | + | In [[Earth science]], chemical [[weathering]] is attributed to [[organic]] chelating agents, such as [[peptide]]s and [[sugar]]s, that extract metal ions from [[mineral]]s and [[rock]]s.<ref>Michael Pidwirny, [http://www.physicalgeography.net/fundamentals/10r.html Introduction to the Lithosphere: Weathering]. ''University of British Columbia Okanagan''. Retrieved December 24, 2008.</ref> Most metal complexes in the environment and in nature are bound in some form of chelate ring, such as with "[[humic acid]]" or a protein. Thus, metal chelates are relevant to the mobilization of [[metals]] in the [[soil]], and the uptake and the accumulation of metals into [[plants]] and [[micro-organisms]]. Selective chelation of [[heavy metal]]s is relevant to [[bioremediation]], such as the removal of <sup>137</sup>Cs from radioactive waste.<ref>Prasad, (ed.) 2001. ''Metals in the Environment: Analysis by Biodiversity.'' University of Hyderabad. (New York: Dekker. ISBN 0824705238.)</ref> |
==Applications== | ==Applications== | ||
| − | Chelators are used in [[chemical analysis]], as [[water softener]]s, and are ingredients in many commercial products such as [[shampoos]] and food [[preservative]]s. [[Citric acid]] is used to [[Citric_acid#Water_softening|soften water]] in [[soap]]s and laundry [[detergent]]s. A common synthetic chelator is [[EDTA]]. [[Phosphonate]]s are also well known chelating agents. Chelators are used in water treatment programs and specifically in [[steam engineering]], | + | Chelators are used in [[chemical analysis]], as [[water softener]]s, and are ingredients in many commercial products such as [[shampoos]] and food [[preservative]]s. [[Citric acid]] is used to [[Citric_acid#Water_softening|soften water]] in [[soap]]s and laundry [[detergent]]s. A common synthetic chelator is [[EDTA]]. [[Phosphonate]]s are also well known chelating agents. Chelators are used in water treatment programs and specifically in [[steam engineering]], such as the [[boiler water treatment system]], or the ''Chelant Water Treatment system.'' |
===Heavy metal detoxification=== | ===Heavy metal detoxification=== | ||
{{main|Chelation therapy}} | {{main|Chelation therapy}} | ||
| − | Chelation therapy is the use of chelating agents to detoxify [[poison]]ous metal agents such as [[mercury poisoning|mercury]], [[arsenic]], and [[lead]] by converting them to a chemically inert form that can be excreted without further interaction with the body | + | Chelation therapy is the use of chelating agents to [[toxicity|detoxify]] [[poison]]ous metal agents such as [[mercury poisoning|mercury]], [[arsenic]], and [[lead]] by converting them to a chemically inert form that can be excreted without further interaction with the human body. This type of therapy was approved by the U.S. Federal Drug Administration [[FDA]] in 1991. Chelation is also used but unproven as a [[Autism therapies#Chelation therapy|treatment]] for [[autism]].<ref>A. Doja and W. Roberts. Immunizations and autism: a review of the literature. ''Can J Neurol Sci'' 2006. 33 (4):341–346 pmid=17168158}}</ref> |
| − | + | Although chelating agents can be beneficial, they can also be dangerous under certain circumstances. For instance, the U.S. CDC reports that use of Na<sub>2</sub>EDTA instead of CaEDTA has resulted in fatalities due to [[hypocalcemia]].<ref>[http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5508a3.htm Deaths Associated with Hypocalcemia from Chelation Therapy]. ''U.S. Centers for Disease Control'', ''MMWR Weekly,'' March 3, 2006. Retrieved December 24, 2008.</ref> | |
===Other medical applications=== | ===Other medical applications=== | ||
| − | [[Antibiotic]] [[medication|drug]]s of the [[tetracycline]] family are chelators of [[Calcium|Ca]]<sup>2+</sup> and [[Magnesium|Mg]]<sup>2+</sup> ions. | + | [[Antibiotic]] [[medication|drug]]s of the [[tetracycline]] family are chelators of [[Calcium|Ca]]<sup>2+</sup> and [[Magnesium|Mg]]<sup>2+</sup> ions. |
| − | EDTA is also used in [[endodontic therapy|root canal treatment]] as a way to irrigate the canal. EDTA softens the dentin facilitating access to the entire canal length and to remove the smear layer formed during instrumentation. | + | EDTA is also used in [[endodontic therapy|root canal treatment]] as a way to irrigate the canal. EDTA softens the dentin, facilitating access to the entire canal length and to remove the smear layer formed during instrumentation. |
[[Gadolinium]](III) compounds and chelates are often used as [[contrast medium|contrast agent]]s in [[MRI|MRI scan]]s. | [[Gadolinium]](III) compounds and chelates are often used as [[contrast medium|contrast agent]]s in [[MRI|MRI scan]]s. | ||
| Line 74: | Line 79: | ||
==References== | ==References== | ||
| − | * Astruc, Didier. 2007. ''Organometallic Chemistry and Catalysis.'' Berlin: Springer. ISBN 9783540461289 | + | * Astruc, Didier. 2007. ''Organometallic Chemistry and Catalysis.'' Berlin: Springer. ISBN 9783540461289. |
| − | * Crabtree, Robert H. 2005. ''The Organometallic Chemistry of the Transition Metals'' | + | * Crabtree, Robert H. 2005. ''The Organometallic Chemistry of the Transition Metals,'' 4th ed. Hoboken, NJ: Wiley. ISBN 978-0471662563. |
| − | * McMurry, John. 2004. ''Organic Chemistry'' | + | * Greenwood, N. N., & Earnshaw, A. 1997. ''Chemistry of the Elements,'' 2nd Edn. Oxford, UK: Butterworth-Heinemann. ISBN 0750633654. |
| − | * Solomons, T.W. Graham, and Craig B. Fryhle. 2004. ''Organic Chemistry'' | + | * Lippard, S.J. and J.M. Berg. 1994. ''Principles of Bioinorganic Chemistry.'' Mill Valley, CA: University Science Books. ISBN 0935702733. |
| − | * Zumdahl, Steven S. 2005. ''Chemical Principles'' | + | * McMurry, John. 2004. ''Organic Chemistry,'' 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. |
| + | * Solomons, T.W. Graham, and Craig B. Fryhle. 2004. ''Organic Chemistry,'' 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
| + | * Zumdahl, Steven S. 2005. ''Chemical Principles.'' New York, NY: Houghton Mifflin. ISBN 0618372067. | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Latest revision as of 19:27, 27 December 2008
Chelation is the binding or complexation of a bidentate or multidentate ligand to a substrate. The ligand, which is often an organic compound, is called a chelant, chelator, chelating agent, or sequestering agent. The substrate is usually a metal ion. The complex formed between the ligand and the substrate is called a chelate complex. The term chelation is reserved for complexes in which the metal ion is bound to two or more atoms of the chelating agent. Common chelators include citric acid, EDTA, and phosphonates.
In nature, various proteins, polysaccharides, and nucleic acids are good chelators of many metal ions. In addition, metal chelates are important for the mobilization of metals in the soil, and the uptake of metals by plants and microorganisms.
Chelation is useful for various practical applications. For example, chelators are used in chemical analysis, as water softeners, as ingredients in shampoos and food preservatives, and in water treatment programs. In medicine, chelating agents may be used to detoxify a person from poisonous metals by converting the metals to chemically inert forms. Some advocate the use of chelation as a treatment for autism. Tetracycline antibiotics are chelators of Ca2+ and Mg2+ ions. EDTA is used in root canal treatment, and gadolinium(III) chelates are used as contrast agents in MRI scans. Although chelating agents can be beneficial, some can be dangerous under certain circumstances.
History and etymology
Chelation is from Greek χηλή, chelè, meaning "claw". The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or chele (Greek) of the lobster or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings."[1]
The Chelate Effect
Consider the two equilibria, in aqueous solution, between the copper(II) ion, Cu2+ and ethylenediamine (en) on the one hand and methylamine, MeNH2 on the other.
In (1) the bidentate ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two monodentate methylamine ligands of approximately the same donor power, meaning that the enthalpy of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the EDTA complex, which has six chelate rings, is much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon of the chelate effect is a firmly established empirical fact.
The thermodynamic approach to explaining the chelate effect considers the equilibrium constant for the reaction: the larger the equilibrium constant, the higher the concentration of the complex.
- [Cu(en] =β11[Cu][en]
- [Cu(MeNH2)2]= β12[Cu][MeNH2]2
Electrical charges have been omitted for simplicity of notation. The square brackets indicate concentration, and the subscripts to the stability constants, β, indicate the stoichiometry of the complex. When the analytical concentration of methylamine is twice that of ethylenediamine and the concentration of copper is the same in both reactions, the concentration [Cu(en)] is much higher than the concentration [Cu(MeNH2)2] because β11 >> β12.
An equilibrium constant, K, is related to the standard Gibbs free energy, ΔG![]() by
by
where R is the gas constant and T is the temperature in Kelvin. ΔH![]() is the standard enthalpy change of the reaction and ΔSImage:StrikeO.png is the standard entropy change. It has already been posited that the enthalpy term should be approximately the same for the two reactions. Therefore the difference between the two stability constants is due to the entropy term. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.[2]
is the standard enthalpy change of the reaction and ΔSImage:StrikeO.png is the standard entropy change. It has already been posited that the enthalpy term should be approximately the same for the two reactions. Therefore the difference between the two stability constants is due to the entropy term. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.[2]
Equilibrium log β ΔG 
ΔHImage:StrikeO.png /kJ mol−1 −TΔSImage:StrikeO.png /kJ mol−1 Cd2+ + 4 MeNH2  Cd(MeNH2)42+
Cd(MeNH2)42+
6.55 -37.4 -57.3 19.9 Cd2+ + 2 en  Cd(en)22+
Cd(en)22+
10.62 -60.67 -56.48 -4.19
These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favorable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy.
Other explanations, including that of Schwarzenbach,[3] are discussed in Greenwood and Earnshaw, 910 (loc.cit).
Chelation in Nature
Many biochemicals exhibit the ability to dissolve certain metal cations. For example, proteins, polysaccharides, and nucleic acids are excellent polydentate ligands for many metal ions. Histidine, malate, and phytochelatin are typical chelators used by plants.[4][5][6]
In biochemistry and microbiology
Virtually all metalloenzymes feature metals that are chelated, usually to peptides or cofactors and prosthetic groups.[7] Such chelating agents include the porphyrin rings in hemoglobin and chlorophyll. Many microbial species produce water-soluble pigments that serve as chelating agents, termed siderophores. For example, species of Pseudomonas are known to secrete pycocyanin and pyoverdin that bind iron. Enterobactin, produced by E. coli, is the strongest chelating agent known.
In geology
In Earth science, chemical weathering is attributed to organic chelating agents, such as peptides and sugars, that extract metal ions from minerals and rocks.[8] Most metal complexes in the environment and in nature are bound in some form of chelate ring, such as with "humic acid" or a protein. Thus, metal chelates are relevant to the mobilization of metals in the soil, and the uptake and the accumulation of metals into plants and micro-organisms. Selective chelation of heavy metals is relevant to bioremediation, such as the removal of 137Cs from radioactive waste.[9]
Applications
Chelators are used in chemical analysis, as water softeners, and are ingredients in many commercial products such as shampoos and food preservatives. Citric acid is used to soften water in soaps and laundry detergents. A common synthetic chelator is EDTA. Phosphonates are also well known chelating agents. Chelators are used in water treatment programs and specifically in steam engineering, such as the boiler water treatment system, or the Chelant Water Treatment system.
Heavy metal detoxification
Chelation therapy is the use of chelating agents to detoxify poisonous metal agents such as mercury, arsenic, and lead by converting them to a chemically inert form that can be excreted without further interaction with the human body. This type of therapy was approved by the U.S. Federal Drug Administration FDA in 1991. Chelation is also used but unproven as a treatment for autism.[10]
Although chelating agents can be beneficial, they can also be dangerous under certain circumstances. For instance, the U.S. CDC reports that use of Na2EDTA instead of CaEDTA has resulted in fatalities due to hypocalcemia.[11]
Other medical applications
Antibiotic drugs of the tetracycline family are chelators of Ca2+ and Mg2+ ions.
EDTA is also used in root canal treatment as a way to irrigate the canal. EDTA softens the dentin, facilitating access to the entire canal length and to remove the smear layer formed during instrumentation.
Gadolinium(III) compounds and chelates are often used as contrast agents in MRI scans.
See also
- Complex (chemistry)
- EDTA
- Functional group
- Inorganic chemistry
- Ligand
- Organic chemistry
- Organometallic chemistry
Notes
- ↑ Gilbert T. Morgan and Harry D. K. Drew. CLXII.—Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. J. Chem. Soc., Trans. 117 (1920): 1456. doi 10.1039/CT9201701456
- ↑ N.N. Greenwood, & A. Earnshaw. 1997. Chemistry of the Elements, 2nd Ed. (Oxford, UK: Butterworth-Heinemann. ISBN 0750633654), 910
- ↑ G. Schwarzenbach. 1952. Der Chelateffekt. Helv. Chim. Acta 35:2344–2359. doi 10.1002/hlca.19520350721
- ↑ U. Krämer, J.D. Cotter-Howells, J M Charnock, A H J M Baker, and J.A.C. Smith. 1996. Free histidine as a metal chelator in plants that accumulate nickel. Nature 379:635–638. doi 10.1038/379635a0.
- ↑ Jurandir Vieira Magalhaes, 2006. Aluminum tolerance genes are conserved between monocots and dicots. Proc. Natl. Acad. Sci. USA 103 (26): 9749-9750. doi=10.1073/pnas.0603957103. pmid=16785425. Retrieved December 24, 2008.
- ↑ Suk-Bong Ha, Aaron P. Smith, Ross Howden, Wendy M. Dietrich, Sarah Bugg, Matthew J. O'Connell, Peter B. Goldsbrough, and Christopher S. Cobbett. 1999. Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 11:1153–1164. doi=10.1105/tpc.11.6.1153. pmid=10368185. Retrieved December 24, 2008.
- ↑ S.J. Lippard and J.M. Berg. 1994. Principles of Bioinorganic Chemistry. (Mill Valley, CA: University Science Books. ISBN 0935702733.)
- ↑ Michael Pidwirny, Introduction to the Lithosphere: Weathering. University of British Columbia Okanagan. Retrieved December 24, 2008.
- ↑ Prasad, (ed.) 2001. Metals in the Environment: Analysis by Biodiversity. University of Hyderabad. (New York: Dekker. ISBN 0824705238.)
- ↑ A. Doja and W. Roberts. Immunizations and autism: a review of the literature. Can J Neurol Sci 2006. 33 (4):341–346 pmid=17168158}}
- ↑ Deaths Associated with Hypocalcemia from Chelation Therapy. U.S. Centers for Disease Control, MMWR Weekly, March 3, 2006. Retrieved December 24, 2008.
ReferencesISBN links support NWE through referral fees
- Astruc, Didier. 2007. Organometallic Chemistry and Catalysis. Berlin: Springer. ISBN 9783540461289.
- Crabtree, Robert H. 2005. The Organometallic Chemistry of the Transition Metals, 4th ed. Hoboken, NJ: Wiley. ISBN 978-0471662563.
- Greenwood, N. N., & Earnshaw, A. 1997. Chemistry of the Elements, 2nd Edn. Oxford, UK: Butterworth-Heinemann. ISBN 0750633654.
- Lippard, S.J. and J.M. Berg. 1994. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 0935702733.
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Solomons, T.W. Graham, and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
- Zumdahl, Steven S. 2005. Chemical Principles. New York, NY: Houghton Mifflin. ISBN 0618372067.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.