Aniline
| Aniline | |
|---|---|
| |
| General | |
| Other names | Phenylamine Aminobenzene |
| Molecular formula | C6H7N |
| SMILES | NC1=CC=CC=C1 |
| Molar mass | 93.13 g/mol |
| Appearance | colorless liquid |
| CAS number | [62-53-3] |
| Properties | |
| Density and phase | 1.0217 g/ml, liquid |
| Solubility in water | 3.6 g/100 mL at 20°C |
| Solubility in ethanol, acetone | Miscible |
| Melting point | −6.3 °C |
| Boiling point | 184.13 °C |
| Basicity (pKb) | 9.40 |
| Viscosity | 3.71 cP at 25 °C |
| Thermodynamic data | |
| Standard enthalpy of formation ΔfH |
? kJ/mol |
| Standard enthalpy of combustion ΔcH |
-3394 kJ/mol |
| Standard molar entropy S |
? J.K−1.mol−1 |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Toxic (T) Carc. Cat. 3 Muta. Cat. 3 Dangerous for the environment (N) |
| NFPA 704 | |
| R-phrases | R23/24/25, R40, R41, R43, R48/23/24/25, R68, R50 |
| S-phrases | S1/2, S26, S27, S36/37/39, S45, S46, S61, S63 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Regulatory data | Flash point, RTECS number, etc. |
| Related compounds | |
| Related aromatic amines | 1-Naphthylamine 2-Naphthylamine |
| Related compounds | Phenylhydrazine Nitrosobenzene Nitrobenzene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
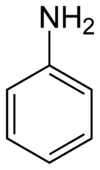
Aniline, phenylamine, or aminobenzene is an organic compound with the formula C6H5NH2. It is an organic chemical compound, specifically an aryl amine, consisting of a phenyl group attached to an amino group. The chemical structure of aniline is shown at the right. It is now used mainly in the manufacture of polyurethane, although it previously was mainly used more for dyes and drugs.
Production
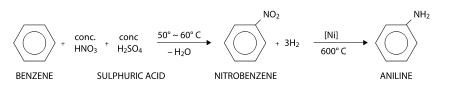
Aniline is produced industrially in two steps from benzene:
First, benzene is heated with a concentrated mixture of nitric acid and sulfuric acid at 50 - 60 °C, where one hydrogen atom is displaced to give nitrobenzene. In this nitration reaction, nitric acid first reacts with suphuric acid giving the electrophile +NO2 which is attracted towards the π-electron cloud of benzene. The +NO2 electrophile attacks the carbon atom, displacing a proton H+ from that particular carbon atom. Nitration is thus called an electrophilic substitution reaction.
Now a mixture of hydrogen gas and nitrobenzene vapors are heated at 600 °C in presence of a nickel catalyst. This gives aniline by reduction. Aniline obtained here is in the pure state.
Many derivatives of aniline can be prepared similarly. In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine, which contains aniline and ortho-toluidine, and is obtained from the distillate (échappés) of the fuchsine fusion. Monomethyl and dimethyl aniline are colorless liquids prepared by heating aniline, aniline hydro-chloride and methyl alcohol in an autoclave at 220 °C. They are of great importance in the color industry. Monomethyl aniline boils at 193-195 °C, dimethyl aniline at 192 °C.
Properties
Aniline is oily and, although colorless, it slowly oxidizes and resinifies in air, giving the sample a red-brown tint.
Like most volatile amines, it possesses a somewhat unpleasant odor of rotten fish, and also has a burning aromatic taste— and it is a highly acrid poison. It ignites readily, burning with a smoky flame.
Chemically, aniline is a weak base. Aromatic amines such as aniline are generally much weaker bases than aliphatic amines. Aniline reacts with strong acids to form anilinium (or phenylammonium) ion (C6H5-NH3+), and reacts with acyl halides such as acetyl chloride to form amides. The amides formed from aniline are sometimes called anilides, for example CH3-CO-NH-C6H5 is acetanilide.
The sulfate forms beautiful white plates. Although aniline is weakly basic, it precipitates zinc, aluminium and ferric salts, and on warming expels ammonia from its salts. Aniline combines directly with alkyl iodides to form secondary and tertiary amines. Boiled with carbon disulfide, it gives sulfocarbanilide (diphenyl thiourea), CS(NHC6H5)2, which may be decomposed into phenyl isothiocyanate, C6H5CNS, and triphenyl guanidine, C6H5N=C(NHC6H5)2. Reaction with sulfuric acid at 180° C produces sulfanilic acid, NH2C6H4SO3H. Anilides, compounds in which the amino group is substituted by an acid radical, are prepared by heating aniline with certain acids; antifebrin or acetanilide is thus obtained from acetic acid and aniline. The oxidation of aniline has been carefully investigated. In alkaline solution azobenzene results, while arsenic acid produces the violet-coloring matter violaniline. Chromic acid converts it into quinone, while chlorates, in the presence of certain metallic salts (especially of vanadium), give aniline black. Hydrochloric acid and potassium chlorate give chloranil. Potassium permanganate in neutral solution oxidizes it to nitrobenzene, in alkaline solution to azobenzene, ammonia and oxalic acid, in acid solution to aniline black. Hypochlorous acid gives 4-aminophenol and para-amino diphenylamine.
Like phenols, aniline derivatives are highly susceptible to electrophilic substitution reactions. For example, sulfonation of aniline produces sulfanilic acid, which can be converted to sulfanilamide. Sulfanilamide is one of the sulfa drugs that were widely used as antibacterials in the early twentieth century.
Aniline and its ring-substituted derivatives react with nitrous acid to form diazonium salts. Through these, the -NH2 group of aniline can be conveniently converted to -OH, -CN, or a halide via Sandmeyer reactions.
It reacts with nitrobenzene to produce phenazine in the Wohl-Aue reaction.
Uses
Originally the great commercial value of aniline was due to the readiness with which it yields, directly or indirectly, valuable dyestuffs. The discovery of mauve in 1856 by William Perkin was the first of a series of dyestuffs which are now to be numbered by hundreds. In addition to its use as a precursor to dyestuffs, it is a starting-product for the manufacture of many drugs such as paracetamol (acetaminophen, Tylenol).
It is used to stain neural RNA blue in the Nissl stain.
Currently the largest market for aniline is the preparation of methylene diphenyl diisocyanate (MDI), some 85 percent of aniline serving this market. Other uses include rubber processing chemicals (nine percent), herbicides (two percent), and dyes and pigments (two percent).[1]
History
Aniline was first isolated from the destructive distillation of indigo in 1826 by Otto Unverdorben, who named it crystalline. In 1834, Friedrich Runge isolated from coal tar a substance which produced a beautiful blue color on treatment with chloride of lime, which he named kyanol or cyanol. In 1841, C. J. Fritzsche showed that by treating indigo with caustic potash it yielded an oil, which he named aniline, from the specific name of one of the indigo-yielding plants, Indigofera anil—anil being derived from the Sanskrit nīla, dark-blue, and nīlā, the indigo plant. About the same time N. N. Zinin found that on reducing nitrobenzene, a base was formed which he named benzidam. August Wilhelm von Hofmann investigated these variously prepared substances, and proved them to be identical (1855), and thenceforth they took their place as one body, under the name aniline or phenylamine.
Its first industrial-scale use was in the manufacture of mauveine, a purple dye discovered in 1856 by William Henry Perkin.
p-Toluidine, an aniline derivative, can be used in qualitative analysis to prepare carboxylic acid derivatives.
Toxicology
Aniline is toxic by inhalation of the vapor, absorption through the skin, or swallowing. It causes headaches, drowsiness, cyanosis, mental confusion and in severe cases can cause convulsions. Prolonged exposure to the vapor or slight skin exposure over a period of time affects the nervous system and the blood, causing tiredness, loss of appetite, headache and dizziness.[2]
Oil mixtures containing rapeseed oil denatured with aniline have been clearly linked by epidemiological and analytic chemical studies to the toxic oil syndrome that hit Spain in the spring and summer of 1981, in which 20,000 became acutely ill, 12,000 were hospitalized, and more than 350 died in the first year of the epidemic. The precise etiology though remains unknown.
Some authorities class aniline as a carcinogen, although the IARC lists it in Group three (not classifiable as to its carcinogenicity to humans) due to the limited and contradictory data available.
See also
Notes
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2
- Solomons, T.W. Graham, and Fryhle, Craig B. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
- This article incorporates text from the Encyclopædia Britannica Eleventh Edition, a publication now in the public domain.
External links
All links retrieved July 27, 2023.
- Toxic Substances Portal - Aniline Agency for Toxic Substances and Disease Registry.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.