Difference between revisions of "Dimethyl sulfoxide" - New World Encyclopedia
(Fixed and changed ending order.) |
|||
| Line 53: | Line 53: | ||
By [[Eric W. Smith]], [[Howard I. Maibach]] Published by CRC Press, 1995 | By [[Eric W. Smith]], [[Howard I. Maibach]] Published by CRC Press, 1995 | ||
ISBN 0849326052, 9780849326059</ref><ref>http://www.medical-library.net/content/view/226/41/ | ISBN 0849326052, 9780849326059</ref><ref>http://www.medical-library.net/content/view/226/41/ | ||
| − | </ref>. This colorless liquid is an important [[polar solvent|polar]] [[aprotic solvent]] that dissolves both polar and nonpolar compounds and is [[miscible]] in a wide range of organic solvents as well as water. | + | </ref>. This colorless liquid is an important [[polar solvent|polar]] [[aprotic solvent]] that dissolves both polar and nonpolar compounds and is [[miscible]] in a wide range of organic solvents as well as water. It has a distinctive property of penetrating the skin very readily, so that one can taste it soon after it comes into contact with the skin. Its taste has been described as [[oyster]]- or [[garlic]]-like. |
==Production== | ==Production== | ||
| − | Dimethyl sulfoxide is a by-product of [[wood pulp]]ing. | + | Dimethyl sulfoxide is a by-product of [[wood pulp]]ing. A supplier of DMSO is the [[Gaylord Chemical Corporation]] in the [[USA]]. |
==Applications== | ==Applications== | ||
| Line 62: | Line 62: | ||
[[Image:Vacuum distillation of DMSO at 70C.jpg|left|thumb|Distillation of DMSO requres a partial vacuum to achieve a lower boiling point.]] | [[Image:Vacuum distillation of DMSO at 70C.jpg|left|thumb|Distillation of DMSO requres a partial vacuum to achieve a lower boiling point.]] | ||
| − | DMSO is an important [[polar aprotic solvent]]. | + | DMSO is an important [[polar aprotic solvent]]. It is less toxic than other members of this class such as [[dimethylformamide]], [[dimethylacetamide]], [[Methylpyrrolidone|''N''-methyl-2-pyrrolidone]], [[Hexamethylphosphoramide|HMPA]]. Because of its excellent solvating power, DMSO is frequently used as a [[solvent]] for chemical reactions involving salts, most notably [[Finkelstein reaction]]s and other [[nucleophilic substitution]]s. Because DMSO is only weakly acidic, it tolerates relatively strong bases, and as such has been extensively used in the study of [[carbanion]]s. A valuable set of non-aqueous [[pKa]] values (C-H, O-H, S-H and N-H acidities) for hundreds of organic compounds have been determined in DMSO solution.<ref name=Bordwell>"Equilibrium acidities in dimethyl sulfoxide solution," F. G. Bordwell [[Acc. Chem. Res.]] '''1988''', ''21'', 456, 463; {{DOI|10.1021/ar00156a004}} [http://www.chem.wisc.edu/areas/reich/pkatable/ Bordwell pKa Table in DMSO]</ref> |
Because of its high boiling point, DMSO evaporates slowly at normal atmospheric pressures. Reactions conducted in DMSO are often diluted with water to precipitate or phase-separate products. DMSO is an effective [[paint stripper]], being safer than many of the others such as [[nitromethane]] and [[dichloromethane]]. The relatively high freezing point of DMSO means that at, or just below, room temperature it is a solid, which can limit its utility in some chemical processes (e.g. [[crystallization]] with cooling). | Because of its high boiling point, DMSO evaporates slowly at normal atmospheric pressures. Reactions conducted in DMSO are often diluted with water to precipitate or phase-separate products. DMSO is an effective [[paint stripper]], being safer than many of the others such as [[nitromethane]] and [[dichloromethane]]. The relatively high freezing point of DMSO means that at, or just below, room temperature it is a solid, which can limit its utility in some chemical processes (e.g. [[crystallization]] with cooling). | ||
| − | In its [[deuterium|deuterated]] form, i.e. [[Deuterated DMSO|DMSO-d<sub>6</sub>]], it is a useful but expensive solvent for [[Nuclear magnetic resonance|NMR]] spectroscopy, again due to its ability to dissolve a wide range of analytes, its own simple spectrum, and its suitability for high-temperature NMR spectroscopic studies. Disadvantages to the use of DMSO-d<sub>6</sub> are its high viscosity, which broadens signals, and high boiling point, which interferes with sample recovery from the NMR solvent. | + | In its [[deuterium|deuterated]] form, i.e. [[Deuterated DMSO|DMSO-d<sub>6</sub>]], it is a useful but expensive solvent for [[Nuclear magnetic resonance|NMR]] spectroscopy, again due to its ability to dissolve a wide range of analytes, its own simple spectrum, and its suitability for high-temperature NMR spectroscopic studies. Disadvantages to the use of DMSO-d<sub>6</sub> are its high viscosity, which broadens signals, and high boiling point, which interferes with sample recovery from the NMR solvent. Often it is mixed with [[deuterated chloroform|CDCl<sub>3</sub>]] or [[deuterated dichloromethane|CD<sub>2</sub>Cl<sub>2</sub>]] for lower viscosity and melting points. |
===Reactions=== | ===Reactions=== | ||
| − | The sulfur center in DMSO is [[nucleophilic]] toward soft [[electrophiles]] and the oxygen is nucleophilic toward hard electrophiles. | + | The sulfur center in DMSO is [[nucleophilic]] toward soft [[electrophiles]] and the oxygen is nucleophilic toward hard electrophiles. The methyl groups of DMSO are somewhat acidic in character (p''K''<sub>a</sub>=35) due to the stabilization of the resultant [[carbanion]] by the S(O)R group, and so are deprotonated with strong bases like [[lithium diisopropylamide]] and [[sodium hydride]]. The sodium salt of DMSO formed in this way (sometimes referred to as "dimsyl sodium") is a useful base, e.g. it is often used for the [[deprotonation]] of [[ketones]] to form sodium [[enolates]], [[phosphonium salt]]s to form [[Wittig reagent]]s, and [[formamidinium]] salts to form [[diaminocarbene]]s. |
DMSO reacts with [[methyl iodide]] to form a sulfoxonium salt [(CH<sub>3</sub>)<sub>3</sub>SO]I, which can be deprotonated with [[sodium]] [[hydride]] to form the [[sulfur]] [[ylide]]: | DMSO reacts with [[methyl iodide]] to form a sulfoxonium salt [(CH<sub>3</sub>)<sub>3</sub>SO]I, which can be deprotonated with [[sodium]] [[hydride]] to form the [[sulfur]] [[ylide]]: | ||
| Line 79: | Line 79: | ||
Products of [[ozonolysis]], [[trioxolane]]s, are quenched with [[dimethyl sulfide]] to produce [[aldehyde]]s and DMSO. | Products of [[ozonolysis]], [[trioxolane]]s, are quenched with [[dimethyl sulfide]] to produce [[aldehyde]]s and DMSO. | ||
| − | DMSO is a ligand for most metal cations. | + | DMSO is a ligand for most metal cations. In the complex [[dichlorotetrakis(dimethyl sulfoxide) ruthenium(II)]], RuCl2(dmso)4, the DMSO [[ligand]] bond to Ru through sulfur and through oxygen. |
===Biology=== | ===Biology=== | ||
| Line 106: | Line 106: | ||
===Medicine=== | ===Medicine=== | ||
| − | In [[cryobiology]] DMSO has been used as a [[cryoprotectant]] and is still an important constituent of cryoprotectant [[vitrification]] mixtures used to preserve organs, tissues, and cell suspensions. Without it, up to 90 percent of frozen cells will become inactive. It is particularly important in the freezing and long-term storage of [[embryonic stem cells]] and [[hematopoietic stem cell]]s, which are often frozen in a mixture of 10% DMSO and 90% [[fetal bovine serum]]. As part of an autologous [[bone marrow transplant]] the DMSO is re-infused along with the patient's own | + | In [[cryobiology]] DMSO has been used as a [[cryoprotectant]] and is still an important constituent of cryoprotectant [[vitrification]] mixtures used to preserve organs, tissues, and cell suspensions. Without it, up to 90 percent of frozen cells will become inactive. It is particularly important in the freezing and long-term storage of [[embryonic stem cells]] and [[hematopoietic stem cell]]s, which are often frozen in a mixture of 10% DMSO and 90% [[fetal bovine serum]]. As part of an autologous [[bone marrow transplant]] the DMSO is re-infused along with the patient's own hematopoietic stem cells. |
| − | Use of DMSO in medicine dates from around | + | Use of DMSO in medicine dates from around 1963, when a [[University of Oregon]] Medical School team, headed by [[Stanley Jacob]], discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system. |
In a 1978 study at the [[Cleveland Clinic]] Foundation in [[Cleveland, Ohio]], researchers concluded that DMSO brought significant relief to the majority of the 213 patients with inflammatory [[genitourinary]] disorders that were studied.<ref>[http://www.dmso.org/articles/bladder/bladder1.htm Dimethyl Sulfoxide in Treatment of Inflammatory Genitourinary Disorders<!-- Bot generated title —>]</ref> They recommended [[DMSO]] for all inflammatory conditions not caused by infection or tumor in which symptoms were severe or patients failed to respond to conventional therapy. | In a 1978 study at the [[Cleveland Clinic]] Foundation in [[Cleveland, Ohio]], researchers concluded that DMSO brought significant relief to the majority of the 213 patients with inflammatory [[genitourinary]] disorders that were studied.<ref>[http://www.dmso.org/articles/bladder/bladder1.htm Dimethyl Sulfoxide in Treatment of Inflammatory Genitourinary Disorders<!-- Bot generated title —>]</ref> They recommended [[DMSO]] for all inflammatory conditions not caused by infection or tumor in which symptoms were severe or patients failed to respond to conventional therapy. | ||
| − | Some people report an onion- or garlic-like taste after touching DMSO. ([[Onion]] and [[garlic]] also derive their odor from sulfoxides [[syn-propanethial-S-oxide]] and [[allicin]].) In the medical field DMSO is predominantly used as a topical [[analgesic]]<ref>{{cite web|author=Diagnose-me.com|title=Topical DMSO|url=http://www.diagnose-me.com/treat/T215027.html|date=2008-04-27|accessdate=2008-06-23}}</ref>, a vehicle for topical application of pharmaceuticals, as an [[anti-inflammatory]]<ref>{{cite web|author=Drugs.com|title=Nuvo announces further update on discussions with the FDA related to review of Pennsaid|url=http://www.drugs.com/nda/pennsaid_070307.html|date=2007-03-27|accessdate=2008-05-01}}</ref> and an [[antioxidant]]{{Fact|date=December 2007}}. It has been examined for the treatment of numerous conditions and ailments. The [[Food and Drug Administration]] (FDA) has approved DMSO usage only for the [[palliative]] treatment of [[interstitial cystitis]]. | + | Some people report an onion- or garlic-like taste after touching DMSO. ([[Onion]] and [[garlic]] also derive their odor from sulfoxides [[syn-propanethial-S-oxide]] and [[allicin]].) In the medical field DMSO is predominantly used as a topical [[analgesic]]<ref>{{cite web|author=Diagnose-me.com|title=Topical DMSO|url=http://www.diagnose-me.com/treat/T215027.html|date=2008-04-27|accessdate=2008-06-23}}</ref>, a vehicle for topical application of pharmaceuticals, as an [[anti-inflammatory]]<ref>{{cite web|author=Drugs.com|title=Nuvo announces further update on discussions with the FDA related to review of Pennsaid|url=http://www.drugs.com/nda/pennsaid_070307.html|date=2007-03-27|accessdate=2008-05-01}}</ref> and an [[antioxidant]]{{Fact|date=December 2007}}. It has been examined for the treatment of numerous conditions and ailments. The [[Food and Drug Administration]] (FDA) has approved DMSO usage only for the [[palliative]] treatment of [[interstitial cystitis]]. Medicinal-grade DMSO for this purpose is manufactured by Insource, Inc. under the name RIMSO. |
Because DMSO increases the rate of absorption of some compounds through organic [[Biological tissue|tissues]] including [[skin]], it can be used as a drug delivery system. | Because DMSO increases the rate of absorption of some compounds through organic [[Biological tissue|tissues]] including [[skin]], it can be used as a drug delivery system. | ||
| − | Dimethyl sulfoxide dissolves a variety of organic substances, including [[carbohydrate]]s, [[polymer]]s, [[peptide]]s, as well as many inorganic salts and gases. Loading levels of 50-60 wt.% are often observed vs 10-20 wt.% with typical solvents. | + | Dimethyl sulfoxide dissolves a variety of organic substances, including [[carbohydrate]]s, [[polymer]]s, [[peptide]]s, as well as many inorganic salts and gases. Loading levels of 50-60 wt.% are often observed vs 10-20 wt.% with typical solvents. For this reason DMSO plays a role in sample management and [[high-throughput screening]] operations in drug design.<ref>{{cite journal | author=Balakin, K. V., Savchuk, N. P., Tetko I. V. | journal=Current Medicinal Chemistry | year=2006 | volume= 13 | issue=2 | title=In silico approaches to prediction of aqueous and DMSO solubility of drug-like compounds: trends, problems and solutions) | doi=10.2174/092986706775197917 | pages=223}}</ref> |
| − | DMSO is commonly used in veterinary medicine as a [[liniment]] for [[horse]]s, alone or in combination with other ingredients. | + | DMSO is commonly used in veterinary medicine as a [[liniment]] for [[horse]]s, alone or in combination with other ingredients. In the latter case, often, the intended function of the DMSO is as a solvent, to carry the other ingredients across the skin. Also in horses, DMSO is used intravenously, again alone or in combination with other drugs. It is used alone for the treatment of increased intracranial pressure and/or cerebral edema in horses. |
====History==== | ====History==== | ||
| − | On September 9, 1965, the ''Wall Street Journal'' reported the death of an Irish woman after undergoing DMSO treatment for a sprained wrist.<ref> Carley W. DMSO May Have Caused Death of Woman, Makers of 'Wonder' Drug Warn Doctors. | + | On September 9, 1965, the ''Wall Street Journal'' reported the death of an Irish woman after undergoing DMSO treatment for a sprained wrist.<ref> Carley W. DMSO May Have Caused Death of Woman, Makers of 'Wonder' Drug Warn Doctors. Wall Street Journal. September 9, 1965:6.</ref> Clinical research using DMSO halted and did not begin again until the National Academy of Sciences (NAS) published findings in favor of DMSO in 1972.{{Fact|date=October 2007}} In 1978, the FDA approved DMSO for treating [[interstitial cystitis]]. In 1980, Congress held hearings on claims that the FDA was slow in approving DMSO for other medical uses. In 2007, the FDA granted "fast track" designation on clinical studies of DMSO's use in reducing brain tissue swelling following traumatic brain injury.{{Fact|date=October 2007}} |
==Safety== | ==Safety== | ||
| − | Glove selection is important when working with DMSO. Thick rubber gloves are recommended. [[Nitrile rubber|Nitrile]] gloves, which are very commonly used in chemical laboratories, have been found to dissolve rapidly with exposure to DMSO.<ref>{{cite web | publisher = [[Cornell University]] | title = Chemical Hygiene Plan | url = http://www.ehs.cornell.edu/geneva/chp/11.glove.selec.htm | date = September 99}}</ref> Because DMSO easily penetrates the skin, substances dissolved in DMSO may be quickly absorbed. | + | Glove selection is important when working with DMSO. Thick rubber gloves are recommended. [[Nitrile rubber|Nitrile]] gloves, which are very commonly used in chemical laboratories, have been found to dissolve rapidly with exposure to DMSO.<ref>{{cite web | publisher = [[Cornell University]] | title = Chemical Hygiene Plan | url = http://www.ehs.cornell.edu/geneva/chp/11.glove.selec.htm | date = September 99}}</ref> Because DMSO easily penetrates the skin, substances dissolved in DMSO may be quickly absorbed. For instance, a solution of [[sodium cyanide]] in DMSO can cause [[cyanide]] poisoning through skin contact. DMSO by itself has low toxicity.<ref>Vignes, Robert (August 2000). [http://www.gaylordchemical.com/bulletins/Vignes-ACS.pdf Dimethyl Sulfoxide (DMSO): A "new" clean, unique, superior solvent], American Chemical Society Annual Meeting</ref> Dimethyl sulfoxide can produce an explosive reaction when exposed to acid chlorides; at a low temperature, this reaction produces the oxidant for [[Swern oxidation]]. |
Recently, it was found that DMSO waste disposal into [[sewers]] can cause environmental odor problems in cities: Waste water bacteria transform DMSO under [[Hypoxia (environmental)|hypoxic]] (anoxic) conditions into [[dimethyl sulfide]] (DMS) that is slightly toxic and has a strong disagreeable odor, similar to rotten cabbage.<ref>{{cite journal | Recently, it was found that DMSO waste disposal into [[sewers]] can cause environmental odor problems in cities: Waste water bacteria transform DMSO under [[Hypoxia (environmental)|hypoxic]] (anoxic) conditions into [[dimethyl sulfide]] (DMS) that is slightly toxic and has a strong disagreeable odor, similar to rotten cabbage.<ref>{{cite journal | ||
| Line 136: | Line 136: | ||
|doi=10.1021/es051312a S0013-936X(05)01312-X }} | |doi=10.1021/es051312a S0013-936X(05)01312-X }} | ||
</ref> | </ref> | ||
| + | |||
| + | {{Urologicals}} | ||
| + | {{Topical products for joint and muscular pain}} | ||
==See also== | ==See also== | ||
| Line 149: | Line 152: | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Revision as of 01:10, 16 November 2008
| Dimethyl sulfoxide | |
|---|---|
| |
|
|
| IUPAC name | Dimethyl sulfoxide |
| Other names | Methyl sulfoxide methylsulfinylmethane DMSO |
| Identifiers | |
| CAS number | [] |
| RTECS number | PV6210000 |
| SMILES | CS(C)=O |
| Properties | |
| Molecular formula | C2H6OS |
| Molar mass | 78.13 g/mol |
| Appearance | Clear, colorless liquid |
| Density | 1.1004 g/cm3, liquid |
| Melting point |
18.5 °C (292 K) |
| Boiling point |
189 °C (462 K) |
| Solubility in water | Miscible |
| Acidity (pKa) | 35 |
| Refractive index (nD) | 1.479 εr = 48 |
| Viscosity | 1.996 cP at 20 °C |
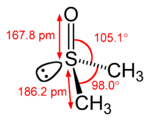
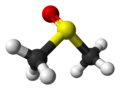
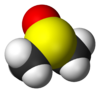
| Structure | |
| Dipole moment | 3.96 D |
| Hazards | |
| MSDS | Oxford MSDS |
| Main hazards | Irritant (Xi), Flammable (F) |
| NFPA 704 |
|
| R-phrases | R36/37/38 |
| S-phrases | S26, S37/39 |
| Flash point | 89 °C |
| Related Compounds | |
| Related sulfoxides | diethyl sulfoxide |
| Related compounds | sodium methylsulfinylmethylide, dimethyl sulfide, dimethyl sulfone, acetone |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Dimethyl sulfoxide (DMSO) is the chemical compound with the formula (CH3)2SO. It was first synthesized in 1866 by the Russian scientist Alexander Saytzeff, who reported his findings in a German chemistry journal in 1867.[1][2]. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a distinctive property of penetrating the skin very readily, so that one can taste it soon after it comes into contact with the skin. Its taste has been described as oyster- or garlic-like.
Production
Dimethyl sulfoxide is a by-product of wood pulping. A supplier of DMSO is the Gaylord Chemical Corporation in the USA.
Applications
Solvent
DMSO is an important polar aprotic solvent. It is less toxic than other members of this class such as dimethylformamide, dimethylacetamide, N-methyl-2-pyrrolidone, HMPA. Because of its excellent solvating power, DMSO is frequently used as a solvent for chemical reactions involving salts, most notably Finkelstein reactions and other nucleophilic substitutions. Because DMSO is only weakly acidic, it tolerates relatively strong bases, and as such has been extensively used in the study of carbanions. A valuable set of non-aqueous pKa values (C-H, O-H, S-H and N-H acidities) for hundreds of organic compounds have been determined in DMSO solution.[3]
Because of its high boiling point, DMSO evaporates slowly at normal atmospheric pressures. Reactions conducted in DMSO are often diluted with water to precipitate or phase-separate products. DMSO is an effective paint stripper, being safer than many of the others such as nitromethane and dichloromethane. The relatively high freezing point of DMSO means that at, or just below, room temperature it is a solid, which can limit its utility in some chemical processes (e.g. crystallization with cooling).
In its deuterated form, i.e. DMSO-d6, it is a useful but expensive solvent for NMR spectroscopy, again due to its ability to dissolve a wide range of analytes, its own simple spectrum, and its suitability for high-temperature NMR spectroscopic studies. Disadvantages to the use of DMSO-d6 are its high viscosity, which broadens signals, and high boiling point, which interferes with sample recovery from the NMR solvent. Often it is mixed with CDCl3 or CD2Cl2 for lower viscosity and melting points.
Reactions
The sulfur center in DMSO is nucleophilic toward soft electrophiles and the oxygen is nucleophilic toward hard electrophiles. The methyl groups of DMSO are somewhat acidic in character (pKa=35) due to the stabilization of the resultant carbanion by the S(O)R group, and so are deprotonated with strong bases like lithium diisopropylamide and sodium hydride. The sodium salt of DMSO formed in this way (sometimes referred to as "dimsyl sodium") is a useful base, e.g. it is often used for the deprotonation of ketones to form sodium enolates, phosphonium salts to form Wittig reagents, and formamidinium salts to form diaminocarbenes.
DMSO reacts with methyl iodide to form a sulfoxonium salt [(CH3)3SO]I, which can be deprotonated with sodium hydride to form the sulfur ylide:
- (CH3)2SO + CH3I → [(CH3)3SO]I
- [(CH3)3SO]I + NaH → [(CH3)2CH2SO + NaI + H2
In organic synthesis, DMSO is used as a mild oxidant,[4] such as the Pfitzner-Moffatt oxidation and the Swern oxidation.[5]
Products of ozonolysis, trioxolanes, are quenched with dimethyl sulfide to produce aldehydes and DMSO.
DMSO is a ligand for most metal cations. In the complex dichlorotetrakis(dimethyl sulfoxide) ruthenium(II), RuCl2(dmso)4, the DMSO ligand bond to Ru through sulfur and through oxygen.
Biology
DMSO is used in PCR to inhibit secondary structures in the DNA template or the DNA primers. It is added to the PCR mix before reacting, where it interferes with the self-complementarity of the DNA, preventing the occurrence of interfering reactions.[6] However, use of DMSO in PCR increases the mutation rate.[citation needed]
DMSO also sees use as a cryoprotectant, added to cell media in order to prevent the cells dying as they are frozen.[7] Approximately 10% may be used with a slow-freeze method, and the cells may be frozen at -20°C or stored in liquid nitrogen safely.
Medicine
In cryobiology DMSO has been used as a cryoprotectant and is still an important constituent of cryoprotectant vitrification mixtures used to preserve organs, tissues, and cell suspensions. Without it, up to 90 percent of frozen cells will become inactive. It is particularly important in the freezing and long-term storage of embryonic stem cells and hematopoietic stem cells, which are often frozen in a mixture of 10% DMSO and 90% fetal bovine serum. As part of an autologous bone marrow transplant the DMSO is re-infused along with the patient's own hematopoietic stem cells.
Use of DMSO in medicine dates from around 1963, when a University of Oregon Medical School team, headed by Stanley Jacob, discovered it could penetrate the skin and other membranes without damaging them and could carry other compounds into a biological system.
In a 1978 study at the Cleveland Clinic Foundation in Cleveland, Ohio, researchers concluded that DMSO brought significant relief to the majority of the 213 patients with inflammatory genitourinary disorders that were studied.[8] They recommended DMSO for all inflammatory conditions not caused by infection or tumor in which symptoms were severe or patients failed to respond to conventional therapy.
Some people report an onion- or garlic-like taste after touching DMSO. (Onion and garlic also derive their odor from sulfoxides syn-propanethial-S-oxide and allicin.) In the medical field DMSO is predominantly used as a topical analgesic[9], a vehicle for topical application of pharmaceuticals, as an anti-inflammatory[10] and an antioxidant[citation needed]. It has been examined for the treatment of numerous conditions and ailments. The Food and Drug Administration (FDA) has approved DMSO usage only for the palliative treatment of interstitial cystitis. Medicinal-grade DMSO for this purpose is manufactured by Insource, Inc. under the name RIMSO.
Because DMSO increases the rate of absorption of some compounds through organic tissues including skin, it can be used as a drug delivery system.
Dimethyl sulfoxide dissolves a variety of organic substances, including carbohydrates, polymers, peptides, as well as many inorganic salts and gases. Loading levels of 50-60 wt.% are often observed vs 10-20 wt.% with typical solvents. For this reason DMSO plays a role in sample management and high-throughput screening operations in drug design.[11]
DMSO is commonly used in veterinary medicine as a liniment for horses, alone or in combination with other ingredients. In the latter case, often, the intended function of the DMSO is as a solvent, to carry the other ingredients across the skin. Also in horses, DMSO is used intravenously, again alone or in combination with other drugs. It is used alone for the treatment of increased intracranial pressure and/or cerebral edema in horses.
History
On September 9, 1965, the Wall Street Journal reported the death of an Irish woman after undergoing DMSO treatment for a sprained wrist.[12] Clinical research using DMSO halted and did not begin again until the National Academy of Sciences (NAS) published findings in favor of DMSO in 1972.[citation needed] In 1978, the FDA approved DMSO for treating interstitial cystitis. In 1980, Congress held hearings on claims that the FDA was slow in approving DMSO for other medical uses. In 2007, the FDA granted "fast track" designation on clinical studies of DMSO's use in reducing brain tissue swelling following traumatic brain injury.[citation needed]
Safety
Glove selection is important when working with DMSO. Thick rubber gloves are recommended. Nitrile gloves, which are very commonly used in chemical laboratories, have been found to dissolve rapidly with exposure to DMSO.[13] Because DMSO easily penetrates the skin, substances dissolved in DMSO may be quickly absorbed. For instance, a solution of sodium cyanide in DMSO can cause cyanide poisoning through skin contact. DMSO by itself has low toxicity.[14] Dimethyl sulfoxide can produce an explosive reaction when exposed to acid chlorides; at a low temperature, this reaction produces the oxidant for Swern oxidation.
Recently, it was found that DMSO waste disposal into sewers can cause environmental odor problems in cities: Waste water bacteria transform DMSO under hypoxic (anoxic) conditions into dimethyl sulfide (DMS) that is slightly toxic and has a strong disagreeable odor, similar to rotten cabbage.[15]
| |||||||||||||||||||||||||
| ||||||||||||||
See also
- Dimethyl sulfide (DMS), the corresponding sulfide
- Dimethyl sulfate (also DMS), the corresponding sulfate: a mutagenic alkylating compound
- Methylsulfonylmethane (MSM), a related chemical often used as a dietary supplement
- Sulfur
Notes
- ↑ "Percutaneous Penetration Enhancers" By Eric W. Smith, Howard I. Maibach Published by CRC Press, 1995 ISBN 0849326052, 9780849326059
- ↑ http://www.medical-library.net/content/view/226/41/
- ↑ "Equilibrium acidities in dimethyl sulfoxide solution," F. G. Bordwell Acc. Chem. Res. 1988, 21, 456, 463; DOI:10.1021/ar00156a004 Bordwell pKa Table in DMSO
- ↑ Epstein W.W., Sweat F.W. (1967). Dimethyl Sulfoxide Oxidations. Chemical Reviews 67: 247–260.
- ↑ Tidwell, T.T. (1990). Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update. Synthesis 1990: 857–870.
- ↑ Chakrabarti R., Schutt C.E. (2001), "The enhancement of PCR amplification by low molecular-weight sulfones", Gene 274 (1-2): 293–298, DOI:10.1016/S0378-1119(01)00621-7
- ↑ Pegg, D.E. (2007), "Principles of cryopreservation", Methods Mol Biol 368: 39-57
- ↑ Dimethyl Sulfoxide in Treatment of Inflammatory Genitourinary Disorders
- ↑ Diagnose-me.com (2008-04-27). Topical DMSO. Retrieved 2008-06-23.
- ↑ Drugs.com (2007-03-27). Nuvo announces further update on discussions with the FDA related to review of Pennsaid. Retrieved 2008-05-01.
- ↑ Balakin, K. V., Savchuk, N. P., Tetko I. V. (2006). In silico approaches to prediction of aqueous and DMSO solubility of drug-like compounds: trends, problems and solutions). Current Medicinal Chemistry 13 (2): 223.
- ↑ Carley W. DMSO May Have Caused Death of Woman, Makers of 'Wonder' Drug Warn Doctors. Wall Street Journal. September 9, 1965:6.
- ↑ Chemical Hygiene Plan. Cornell University (September 99).
- ↑ Vignes, Robert (August 2000). Dimethyl Sulfoxide (DMSO): A "new" clean, unique, superior solvent, American Chemical Society Annual Meeting
- ↑ Glindemann, D., Novak, J., Witherspoon, J. (2006). Dimethyl Sulfoxide (DMSO) Waste Residues and Municipal Waste Water Odor by Dimethyl Sulfide (DMS): the North-East WPCP Plant of Philadelphia. Environmental Science and Technology 40 (1): 202–207.
ReferencesISBN links support NWE through referral fees
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.

