Difference between revisions of "Tyrosine" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 75: | Line 75: | ||
where ''R'' represents a ''side chain'' specific to each amino acid. | where ''R'' represents a ''side chain'' specific to each amino acid. | ||
| − | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In | + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In tyrosine, only the L-stereoisomer is involved in synthesis of [[mammal]]ian proteins. |
| − | + | Tyrosine's chemical formula is C<sub>9</sub>H<sub>11</sub>NO<sub>3</sub> (IUPAC-IUB 1983) (that is, one more nitrogen atom than phenylalanine). | |
| − | Like [[ | + | Like [[phenylalanine ]] and [[tryptophan]], tyrosine contains a large rigid aromatic group on the side chain; in the case of tyrosine, a [[phenol]] side chain with a [[hydroxyl]] group. Tyrosine, phenylalanine, and tryptophan—like [[isoleucine]], [[leucine]], and [[valine]]—are hydrophobic and tend to orient towards the interior of the folded protein molecule. |
| − | + | ===Isomers=== | |
| + | [[Image:Phe_Tyr.jpg|right|250px|tyrosine molecule]] | ||
| + | Based on the location of the hydroxyl group on the side chain, there are three structural isomers of tyrosine, namely para-tyrosine (p-Tyr), meta-tyrosine (m-Tyr), and ortho-tyrosine (o-Tyr). Enzymatically, only the first isomer (p-Tyr) is produced from L-phenylalanine by the phenylalanine-hydroxylase enzyme. The other two isoforms, m-Tyr and o-Tyr, can be produced as a consequence of free radical attack on Phe in states with increased oxidative stress. | ||
| + | |||
| + | == Biosynthesis == | ||
| + | Tyrosine cannot be completely synthesized by animals, although it can be made by hydroxylation of [[phenylalanine]] if the latter is in abundant supply. It is produced by plants and most microorganisms from [[Prephenic acid|prephenate]], an intermediate on the [[Shikimic acid|shikimate pathway]]. | ||
| + | |||
| + | Prephenate is [[Oxidation|oxidatively]] [[Decarboxylation|decarboxylated]] with retention of the [[hydroxyl]] group to give ''p''-hydroxyphenylpyruvate. This is [[Transamination|transaminated]] using [[Glutamic acid|glutamate]] as the nitrogen source to give tyrosine and [[Ketoglutaric acid|α-ketoglutarate]]. | ||
| + | |||
| + | ==Biological aspects== | ||
| + | |||
| + | L-phenylalanine can be converted into L-[[tyrosine]], utilizing the enzyme [[phenylalanine hydroxylase]]. In turn, L-tyrosine is converted to [[levodopa]] (L-DOPA) by the enzyme tyrosine hydroxylase. This can be further converted into [[dopamine]], [[norepinephrine]] (noradrenaline), and [[epinephrine]] (adrenaline) (the latter three are known as [[catecholamine]]s). | ||
| + | |||
| + | [[Image:Tyrosine biosynthesis.png|450px]] | ||
| − | |||
| − | |||
Some of the tyrosine residues can be ''tagged'' with a phosphate group ([[phosphorylation|phosphorylated]]) by [[protein kinase]]s. (In its phosphorylated state, it is referred to as '''phosphotyrosine'''.). Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as [[tyrosine sulfation]]. [[Tyrosine sulfation]] is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine. | Some of the tyrosine residues can be ''tagged'' with a phosphate group ([[phosphorylation|phosphorylated]]) by [[protein kinase]]s. (In its phosphorylated state, it is referred to as '''phosphotyrosine'''.). Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as [[tyrosine sulfation]]. [[Tyrosine sulfation]] is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine. | ||
| Line 91: | Line 102: | ||
In [[Papaver somniferum]], the opium poppy, it is used to produce [[morphine]]. | In [[Papaver somniferum]], the opium poppy, it is used to produce [[morphine]]. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
===Metabolic pathways=== | ===Metabolic pathways=== | ||
Revision as of 00:57, 21 June 2007
| Tyrosine | |
|---|---|
| |
| Systematic name | (S)-2-Amino-3-(4-hydroxy- phenyl)-propanoic acid |
| Abbreviations | Tyr Y |
| Chemical formula | C9H11NO3 |
| Molecular mass | 181.19 g mol-1 |
| Melting point | 343 °C |
| Density | 1.456 g cm-3 |
| Isoelectric point | 5.66 |
| pKa | 2.24 9.04 10.10 |
| Molar extinction coefficient | 1420 M-1 cm-1 at 274.6 nm |
| PubChem | 1153 |
| CAS number | [60-18-4] |
| EINECS number | 200-460-4 |
| SMILES | N[C@@H](Cc1ccc(O)cc1)C(O)=O |
Absorption and emission spectrum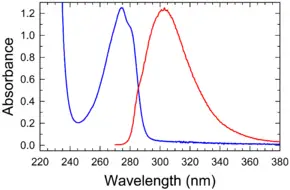
Absorbance and fluorescence of tyrosine in water/buffer | |
| Disclaimer and references | |
Tyrosine is an α-amino acid that is found in most proteins in small amounts, is normally readily converted from the essential amino acid phenylalanine in the human body, and is a precursor of such important chemcial compounds as epinephrine (adrenaline), norepinephrine (noradrenaline), dopamine, thyroxine, and melanin.
In humans, the L-isomer of tyrosine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. However, tyrosine is considered to be a "non-essential amino acid" since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions—in this case, synthesized from phenylalanine. Tyrosine, phenylalanine, and tryptophan are the biggest of the standard amino acids.
The intricate coordination of systems in the human body is seen in the process, catalyzed by enzymes, by which l-phenylalanine is degraded into l-tyrosine, which in turn is converted into L-DOPA, which is further metabolized into other vitally important products: dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). However, in the advent of the lack of a particular enzyme due to a genetic effect, the harmony is disrupted and the body loses its ability to metabolize phenylalanine, resulting in the serious disorder phenylketonuria.
Tyrosine's three letter code is Tyr, its one letter code is Y, and its systematic name is 2-Amino-3-(4-hydroxyphenyl)-propanoic acid (IUPAC-IUB 1983). It is also known as 4-hydroxyphenylalanine.
The name tyrosine is derived from the Greek tyros, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in cheese, obtained as a degradation product of the protein casein.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In tyrosine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Tyrosine's chemical formula is C9H11NO3 (IUPAC-IUB 1983) (that is, one more nitrogen atom than phenylalanine).
Like phenylalanine and tryptophan, tyrosine contains a large rigid aromatic group on the side chain; in the case of tyrosine, a phenol side chain with a hydroxyl group. Tyrosine, phenylalanine, and tryptophan—like isoleucine, leucine, and valine—are hydrophobic and tend to orient towards the interior of the folded protein molecule.
Isomers
Based on the location of the hydroxyl group on the side chain, there are three structural isomers of tyrosine, namely para-tyrosine (p-Tyr), meta-tyrosine (m-Tyr), and ortho-tyrosine (o-Tyr). Enzymatically, only the first isomer (p-Tyr) is produced from L-phenylalanine by the phenylalanine-hydroxylase enzyme. The other two isoforms, m-Tyr and o-Tyr, can be produced as a consequence of free radical attack on Phe in states with increased oxidative stress.
Biosynthesis
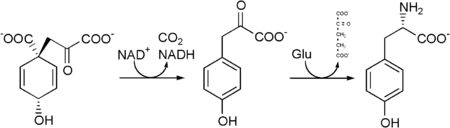
Tyrosine cannot be completely synthesized by animals, although it can be made by hydroxylation of phenylalanine if the latter is in abundant supply. It is produced by plants and most microorganisms from prephenate, an intermediate on the shikimate pathway.
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate. This is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
Biological aspects
L-phenylalanine can be converted into L-tyrosine, utilizing the enzyme phenylalanine hydroxylase. In turn, L-tyrosine is converted to levodopa (L-DOPA) by the enzyme tyrosine hydroxylase. This can be further converted into dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) (the latter three are known as catecholamines).
Some of the tyrosine residues can be tagged with a phosphate group (phosphorylated) by protein kinases. (In its phosphorylated state, it is referred to as phosphotyrosine.). Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as tyrosine sulfation. Tyrosine sulfation is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine. Tyrosine is also precursor to the thyroid hormones thyroxine and triiodothyronine, the pigment melanin, and the biologically-active catecholamines dopamine, norepinephrine and epinephrine.
In Papaver somniferum, the opium poppy, it is used to produce morphine.
Metabolic pathways

The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into the amino acid tyrosine. If this reaction does not take place, phenylalanine accumulates and tyrosine is deficient. Excessive phenylalanine can be metabolized into phenylketones, which are detected in the urine. These include phenylacetate, [[phenylpyruvate] and phenylethylamine[2]. Detection of phenylketones in the urine is diagnostic.
Phenylalanine is a large, neutral amino acid (LNAA). LNAAs compete for transport across the blood brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). Excessive phenylalanine in the blood saturates the transporter. Thus, excessive levels of phenylalanine significantly decrease the levels of other LNAAs in the brain. But since these amino acids are required for protein and neurotransmitter synthesis, phenylalanine accumulation disrupts brain development in children, leading to mental retardation.[3]
Tyrosine hydroxylase
Tyrosine hydroxylase (TH) is the rate-limiting enzyme involved in the synthesis of the catecholamines such as dopamine, norepinephrine and epinephrine.
Medical use
L-Tyrosine is sometimes recommended by practitioners as helpful for weight loss, clinical depression, Parkinson's Disease, and phenylketonuria; however, one study found that it had no impact on endurance exercise performance. [4]
Phenylketonuria
Phenylketonuria (PKU) is an autosomal recessive genetic disorder characterized by a deficiency in the enzyme phenylalanine hydroxylase (PAH). This enzyme is necessary to metabolize the amino acid phenylalanine to the amino acid tyrosine. When PAH is deficient, phenylalanine accumulates and is converted into phenylketones, which are detected in the urine.
Left untreated, this condition can cause problems with brain development, leading to progressive mental retardation and seizures. However, PKU is one of the few genetic diseases that can be controlled by diet. A diet low in phenylalanine and high in tyrosine can bring about a nearly total cure.
Phenylketonuria
The genetic disorder phenylketonuria (PKU) is the inability to metabolize phenylalanine. It is a genetic disorder characterized by a deficiency in the enzyme phenylalanine hydroxylase (PAH), which is necessary to metabolize the phenylalanine to tyrosine. When PAH is deficient, phenylalanine accumulates and is converted into phenylketones, which are detected in the urine. These include phenylacetate, phenylpyruvate, and phenylethylamine (Michals and Matalon 1985). Detection of phenylketones in the urine is diagnostic.
Left untreated, this condition can cause problems with brain development, leading to progressive mental retardation and seizures (see Biological aspects above). However, PKU is one of the few genetic diseases that can be controlled by diet. A diet low in phenylalanine and high in tyrosine can bring about a nearly total cure.
Individuals with this disorder are known as "phenylketonurics." Treatment of PKU includes the elimination of phenylalanine from the diet, and supplementation of the diet with tyrosine. Babies who are diagnosed with PKU must immediately be put on a special milk/formula substitute. Later in life, the diet continues to exclude phenylalanine-containing foods. Women affected by PKU must pay special attention to their diet if they wish to become pregnant, since high levels of phenylalanine in the uterine environment can cause severe malformation and mental retardation in the child. However, women who maintain an appropriate diet can have normal, healthy children. This dietary restriction also applies to pregnant women with hyperphenylalanine (high levels of phenylalanine in blood) because they do not properly metabolize phenylalanine.
If PKU is diagnosed early enough, an affected newborn can grow up with normal brain development, but only by eating a special diet low in phenylalanine for the rest of his or her life. This requires severely restricting or eliminating foods high in phenylalanine, such as breast milk, meat, chicken, fish, nuts, cheese, and other dairy products. Starchy foods such as potatoes, bread, pasta, and corn must be monitored. Many diet foods and diet soft drinks that contain the sweetener aspartame must also be avoided, as aspartame consists of two amino acids: phenylalanine and aspartic acid. Aspartame is found in many sugarless gums, sugarless soft drinks (such as Diet Coke, and Diet Pepsi), some forms of Lipton Tea, and a number of other food products.
ReferencesISBN links support NWE through referral fees
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Michals, K., and R. Matalon. 1985. Phenylalanine metabolites, attention span and hyperactivity. American Journal of Clinical Nutrition. 42(2): 361-365. PMID 4025205.
- Pietz, J., R. Kreis, A. Rupp, E. Mayatepek, D. Rating, C. Boesch, and H. J. Bremer. 1999. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. Journal of Clinical Investigation 103: 1169–1178. PMID 10207169.
References
- AJ Hoffhines et al. Journal of Biological Chemistry 281:37877-37887, 2006[1]
- GA Molnar et al. Kidney International 68:2281-2287, 2005 Abstract
- GA Molnar et al. Free Radical Research 39(12):1359-1366, 2005 Abstract
Notes
- ↑ 1.0 1.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedFolling - ↑ Michals, K., Matalon, R. (1985). Phenylalanine metabolites, attention span and hyperactivity. American Journal of Clinical Nutrition 42(2): 361-365. PMID 4025205.
- ↑ Pietz, J., Kreis, R., Rupp, A., Mayatepek, E., Rating, D., Boesch, C., Bremer, H. J. (1999). Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. Journal of Clinical Investigation 103: 1169–1178. PMID 10207169.
- ↑ Parcell A.C., et al. Effects of L-tyrosine and carbohydrate ingestion on performance. Journal of Applied Physiology 2002 Nov; 93(5): 1590-97. Abstract
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.