Serine
| Serine | |
|---|---|
| |
| Systematic name | (S)-2-amino-3-hydroxypropanoic acid |
| Abbreviations | Ser S |
| Chemical formula | C3H7NO3 |
| Molecular mass | 105.09 g mol-1 |
| Melting point | 228 °C |
| Density | 1.537 g cm-3 |
| Isoelectric point | 5.68 |
| pKa | 2.13 9.05 |
| CAS number | [56-45-1] |
| PubChem | 5951 |
| EINECS number | 200-274-3 |
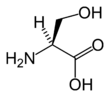
| SMILES | N[C@@H](CO)C(O)=O |
Serine is an α-amino acid that is common in many proteins, sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. Serine is an important component of phospholipids and participates in the biosynthesis of purines and pyrimidines, as well as such amino acids as cysteine and glycine. With an easily removed hydrogen on the hydroxyl side chain, serine is often a hydrogen donor in enzymes, such as trypsin and chymotrypsin, playing an important role in their function as catalysts.
In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be a "non-essential" amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Human creativity, which can be used for good or ill purposes, has exploited serine's role in the active site of the enzyme acetylcholine esterase to produce both nerve gases, such as Sarin that causes painful deaths in humans, and insecticides, which are designed to increase human agricultural productivity and prosperity. (See function below.)
Serine's three letter code is Ser, its one letter code is S, its codons are AGU and AGC, and its systematic name is 2-Amino-3-hydroxypropanoic acid (IUPAC-IUB 1983). The name serine was derived from the Latin for silk, "sericum," since serine was first isolated from silk protein. While the amino acids glycine and alanine make up the bulk of silk protein, it is also a rich source of serine.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acidsâthose amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called αâcarbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In serine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
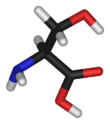
Serine has the chemical formula HO-CH2-CH(NH2)-COOH (alternatively, HO2C-CH(NH2)-CH2-OH), or more generally, C3H7NO3.
Serine, like threonine, has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them.
Biosynthesis
Serine is not essential to the human diet, since it is synthesized in the body. The synthesis of serine starts with the oxidation of 3-phosphoglycerate forming 3-phosphohydroxypyruvate and NADH. Reductive amination of this ketone followed by hydrolysis yields serine.
Serine also gives rise to the amino acid glycine, thus glycine is not classified as an essential amino acid. The enzyme serine hydroxymethyl transferase catalyzes this reversible, simultaneous conversions of L-serine to glycine (retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate (hydrolysis) (Lehninger 2000). The conversion of serine to glycine is characterized as follows:
- HO2C-CH(NH2)-CH2-OH (serine) + H2folate â HO2CCH2NH2 (glycine) + CH2-folate + H2O
Serine is prepared commercially from methyl acrylate (Carter and West 1955).
Function
Serine plays a role in the biosynthesis of proteins, phospholipids, purines, pyrimidines, the amino acids cysteine and glycine, and many other biologically important compounds. It is also the precursor to numerous of other metabolites, including sphingolipids and folate, which is the principal donor of one carbon fragments in biosynthesis.
Like cysteine, serine often helps an enzyme catalyze its reaction, occuring in the active sites of such enzymes as trypsin (a serine protease found in the digestive system, where it breaks down proteins) and chymotrypsin (a digestive enzyme that can perform proteolysis, cleaving peptides at the carboxyl side of tyrosine, tryptophan, and phenylalanine).
As a constituent (residue) of proteins, serine's side chain can undergo O-linked glycosylation. This might be important in explaining some of the devastating consequences of diabetes. It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine. Serine proteases, such as trypsin, are a common type of protease.
Serine's role in the active site of acetylcholine esterase has been exploited in the production of nerve gases, such as Sarin, and insecticides. Acetylcholine is a small organic molecule that serves as an important neurotransmitter, relaying information across the gap (synapse) between a neuron (nerve cell) and an adjacent cell (another neuron or a muscle or gland cell). After acetylcholine has completed its role of transmitting the message (for the electrical impulse to continue in the adjacent neuron, or the muscle cell to contract, or the gland to secrete), it must be removed so that it does not keep stimulating the receptor cell. The enzyme acetylcholine esterase fulfills this function, converting acetylcholine into the inactive metabolites choline and acetate and clearing free acetylcholine from the synapse. It is a fast enzyme that can rapidly hydrolyze acetylcholineâ10,000 molecules of acetylcholine can be hydrolyzed in one second by one molecule of this enzyme.
However, Sarin and other nerve gases combine with a residue (constituent) of serine in the active site and cause irreversible inactivation of this enzyme. The resulting accumulation of acetylcholine causes continuous stimulation of the muscles, glands, and the central nervous system; victims commonly die of suffocation as they cannot contract their diaphragm. Other organophosphates and some carbamates are effective insecticides because they inhibit acetylcholinesterase in insects.
D-serine, synthesized by serine racemase from L-serine, serves as a neuronal signaling molecule by activating NMDA receptors in the brain.
ReferencesISBN links support NWE through referral fees
- Carter, H. E., and H. D. West. dl-Serine Organic Syntheses, 3: 774, 1955. Retrieved September 24, 2007.
- Doolittle, R. F. âRedundancies in protein sequences.â In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press, 1989. ISBN 0306431319
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB, 1983. Retrieved September 24, 2007.
- Kendall, E. C., and B. F. McKenzie. dl-Alanine Organic Syntheses, 1: 21, 1941. Retrieved September 24, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing, 2000. ISBN 1572591536
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.