Arginine
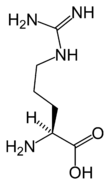  Chemical structure of L-arginine | |
Arginine | |
| Systematic (IUPAC) name | |
| 2-amino-5-(diaminomethylidene amino)pentanoic acid | |
| Identifiers | |
| CAS number | 74-79-3 |
| PubChem        | 6322 |
| Chemical data | |
| Formula | C6H14N4O2Â |
| Mol. weight | 174.2 |
| SMILES | N[C@@H](CCCNC(N)=N)C(O)=O |
| Complete data | |
Arginine is an α-amino acid and the L-form, the only form involved in protein synthesis, one of the 20 most common natural amino acids in proteins.
In mammals, arginine is classified as a semi-essential, or conditionally essential, amino acid, depending on the developmental stage and health status of the individual (Morris 2004). Essential amino acids are those that cannot be synthesized by an animal, or at a rate sufficient to meet its physiological needs, and which therefore must be obtained from the diet. Adult humans can synthesize arginine in sufficient quantities, via the urea cycle. However, infants are unable to effectively synthesize arginine, making it nutritionally essential for infants. Thus, arginine (like histidine) is sometimes classified as essential amino acids, though it is generally considered essential only in children.
Human beings, in their desire to better understand their physical environment, have uncovered much about the amino acid argonine and its important role. Today, it is used in biochemical research, in medicine, and as a dietary supplement. Among its many uses is promoting the healing of wounds, improvement of the immune response, and treating people with chronic heart failure and conditions where vasodilation is required (such as high blood pressure).
Arginine was first isolated from a lupin seedling extract in 1886, by the Swiss chemist Ernst Schulze.
Arginine's three letter code is Arg, its one letter code is R, and its systematic name is 2-Amino-5-guanidinopentanoic acid (IUPAC-IUB 1983), or 2-amino-5-(diaminomethylidene amino)pentanoic acid.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called 뱉carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a "side chain" specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis.
Arginine's chemical formula is H2N-C(=NH)-NH-[CH2]3-CH(NH2)-COOH or in general form C6H14N4O2 (IUPAC-IUB 1983).
Arginine can be considered to be a basic amino acid because the part of the side chain nearest to the backbone is long, carbon-containing, and hydrophobic, whereas the end of the side chain is a complex guanidinium group. With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic, and even most basic environments. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized. This group is able to form multiple H-bonds.
Food sources
Arginine is found in chocolate, wheat germ and flour, buckwheat, granola, oatmeal, dairy products (cottage cheese, ricotta, nonfat dry milk, skim yogurt), beef (roasts, steaks), pork (bacon, ham), nuts (coconut, pecans, cashews, walnuts, almonds, Brazil nuts, hazel nuts, peanuts), seeds (pumpkin, sesame, sunflower), poultry (chicken and turkey light meat), wild game (pheasant, quail), seafood (halibut, lobster, salmon, shrimp, snails, tuna in water), chick peas, cooked soybeans, and some energy drinks.
Synthesis
Arginine is synthesized in the body from the α-amino acid citrulline by the sequential action of the cytosolic enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). This is energetically costly, as the synthesis of each molecule of argininosuccinate requires hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP); that is, two ATP equivalents.
Citrulline can be derived from multiple sources:
- from arginine via nitric oxide synthase (NOS);
- from the amino acid ornithine via catabolism of the amino acids proline or glutamine/glutamate;
- from asymmetric dimethylarginine (ADMA) via DDAH.
The pathways linking arginine, glutamine, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage.
On a whole-body basis, synthesis of arginine occurs principally via the intestinalârenal (intestine-kidney) axis, wherein epithelial cells of the small intestine, which produce citrulline primarily from glutamine and glutamate, collaborate with the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which is returned to the circulation. Consequently, impairment of small bowel or renal function can reduce endogenous arginine synthesis, thereby increasing the dietary requirement.
Synthesis of arginine from citrulline also occurs at a low level in many other cells, and cellular capacity for arginine synthesis can be markedly increased under circumstances that also induce nitric oxide synthase (iNOS). Thus, citrulline, a coproduct of the NOS-catalyzed reaction, can be recycled to arginine in a pathway known as the citrulline-NO or arginine-citrulline pathway. This is demonstrated by the fact that in many cell types, citrulline can substitute for arginine to some degree in supporting NO synthesis. However, recycling is not quantitative because citrulline accumulates along with nitrate and nitrite, the stable end-products of nitric oxide (NO), in NO-producing cells (Morris 2004).
Function
In proteins, the geometry, charge distribution, and ability to form multiple H-bonds make arginine ideal for binding negatively charged groups. For this reason, arginine is preferentially on the outside of the proteins, where it can interact with the polar environment. Incorporated in proteins, arginine can also be converted to citrulline by PAD enzymes. In addition, arginine can be methylated by protein methyltransferases.
Arginine is the immediate precursor of nitric oxide (NO), urea, ornithine, and agmatine. It is necessary for the synthesis of creatine, and can be used for the synthesis of polyamines (mainly through ornithine and to a lesser degree through agmatine), citrulline, and glutamate.
Arginine plays an important role in cell division and removing ammonia from the body.
General health use
Various research indicates that arginine is important for growth periods, but not body maintenance (Longe 2004). Arginine increases the production and release of growth hormone (Alba-Roth et al. 1988). Reports of its effects on male muscular development are not clearly proven.
Arginine is considered to be crucial to the healing of wounds and the improvement of immune system response to bacteria, viruses, and tumor cells (Longe 2004). Its role in promoting liver regeneration allows arginine to be used in treating people with liver disfunction (Longe 2004). It is considered potentially useful for treating people with chronic heart failure (Jalal Al Mosawi 2024).
Arginine, which the body naturally converts into NO, a chemical that relaxes blood vessels, makes arginine of use in many conditions where vasodilation is required. Arginine has been shown to have a vasodilatory effect on people with high blood pressure, and others with compromised circulation problems associated with heart disease (Brodin 2024). It is used as a supplement in treating those with arterial heart disease and for easing exercise-related pains caused by the heart muscle not getting enough blood to circulate to the calf muscles. Arginine, taken in combination with proanthocyanidins (Stanislavov and Nikolova 2003) or yohimbine (Lebret et al. 2002) has also been used as a treatment for erectile dysfunction.
The presence of asymmetric dimethylarginine (ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA is considered a marker for vascular disease, just as L-arginine is considered a sign of a healthy endothelium.
Arginine may have implications in herpes simplex viral replication. Tissues culture studies have shown the suppression of viral replication when the lysine to arginine ratio in vitro favors lysine. The therapeutic consequence of this finding is unclear, but dietary arginine may affect the effectiveness of lysine supplementation (Griffith et al. 1978). Treatment of arginine has also been shown to improve immune function in HIV patients.
Arginine supplements have been considered to be an effective anticoagulate, but unlike aspirin and other anticoagulants, could prevent clotting without increasing stroke risk.
On the other hand, a Johns Hopkins study testing the addition of L-arginine to standard post-infection treatment has implicated L-arginine supplementation with an increased risk of death in patients recovering from heart attack (Schulman et al. 2006).
ReferencesISBN links support NWE through referral fees
- Alba-Roth, J., O. MĂŒller, J. Schopohl, and K. von Werder. 1988. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab 67(6): 1186-1189. Retrieved April 1, 2025.
- Brodin, M. 2024. L-arginine: Does it lower blood pressure?. Mayo Clinic. Retrieved April 1, 2025.
- Griffith, R.S., A.L. Norins, and C. Kagan. 1978. A multicentered study of lysine therapy in Herpes simplex infection. Dermatologica 156(5): 257-267. Retrieved April 1, 2025.* * Jalal Al Mosawi, A. 2024. The use of L-arginine in heart failure: An educational article and expert opinion Clinical Research and Clinical Reports, 3(2). Retrieved April 1, 2025.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved April 1, 2025.
- Lebret, T., J.M. Hervéa, P. Gornyb, M. Worcelc, and H. Botto. 2002. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: A new oral therapy for erectile dysfunction. European Urology 41(6): 608-613. Retrieved April 1, 2025.
- Longe, J.L. (ed.) 2004. The Gale Encyclopedia of Alternative Medicine. Detroit: Thomson/Gale. ISBN 978-0787674243
- Morris, S.M. 2004. Enzymes of arginine metabolism. J Nutr. 134(10 Suppl): 2743S-2747S. Retrieved April 1, 2025.
- Schulman, S.P., L.C. Becker, D.A. Kass, H.C. Champion, M.L. Terrin, S. Forman, K.V. Ernst, M.D. Kelemen, S.N. Townsend, A. Capriotti, J.M. Hare, and G. Gerstenblith. 2006. Arginine therapy in acute myocardial infarction: The vascular interaction with age in myocardial infarction (VINTAGE MI) randomized clinical trial. JAMA 295: 58-64. Retrieved April 1, 2025.
- Stanislavov, R., and V. Nikolova. 2003. Treatment of erectile dysfunction with pycnogenol and L-arginine. Journal of Sex and Marital Therapy 29(3): 207 â 213. Retrieved April 1, 2025.
External links
All links retrieved April 1, 2025.
- L-arginine Mayo Clinic
- L-Arginine Cleveland Clinic
- L-Arginine Medline Plus
- 10 Healthy High-Arginine Foods Healthline
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.