Aspartame
| Aspartame | |
|---|---|
| |
| Chemical name | N-(L-α-Aspartyl)-L-phenylalanine, 1-methyl ester |
| Other names | NutraSweet Canderel Equal |
| Chemical formula | C14H18N2O5 |
| Molecular mass | 294.301 g/mol |
| CAS number | [22839-47-0] |
| Melting point | 246-247 °C |
| Boiling point | decomposes |
| SMILES | |
| NFPA 704 | |
| Disclaimer and references | |
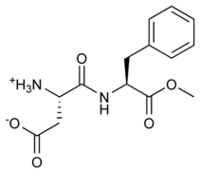
Aspartame (IPA: /ˈæ.spɚˌteɪm/ or /əˈspɑɹˌteɪm/) is the name for a non-carbohydrate, non-nutritive artificial sweetener and flavor enhancer, aspartyl-phenylalanine-1-methyl ester, that is synthesized from two amino acids, aspartic acid and the essential amino acid, phenylalanine. It is often used as a sugar substitute.
Aspartame is 180 to 200 times sweeter than sugar (Herbst 2001) and is marketed under a number of trademark names, such as NutraSweet®, Equal, and Canderel. Aspartame is an ingredient in approximately 6,000 consumer foods and beverages sold worldwide. It is commonly used in diet soft drinks, as a table condiment, and in some brands of chewable vitamin supplements and sugar-free chewing gums. Aspartame is also one of the sugar substitutes used by diabetics. However, aspartame is not always suitable for baking because it often breaks down when heated and loses much of its sweetness, and at temperatures above 90oF a component of it can convert to formaldehyde (Chamberlin and Narins 2005).
Human beings have an attraction to sweet food. Even ancient cave paintings at Arana in Spain exhibit a neolithic man procuring honey from a bee's nest (Blachford 2002). Carbohydrates not only are a vital component needed by humans, but sweet items also address an internal aspect of people, the joy of taste. Thus, sugar (sucrose) is utilized in desserts, placed in coffee and tea, and used in many foods and drinks. However, sweet things also have a lot of calories, thus contributing to problems with obesity, and overconsumption of sucrose has been linked to a number of other deleterious health consequences, including tooth decay and diabetes. Aspartame provides the desired sweetness without high calories and the other known physical characteristics of sugar that adversely affect health. For the same level of sweetness as sugar, a less amount of aspartame (and less calories) is required. Aspartame further synergizes with other sweeteners, allowing use of less total sweetener, and it also intensifies and extends fruit flavors (Blachford 2002).
However, aspartame also is the subject of a small but vigorous public debate due to perceived health risks. It is considered by some scientists and special interest groups to be detrimental to the nervous system, but that allegation remains controversial (Chamberlin and Narins 2005). It has lost market share in recent years to sucralose (Splenda, Altern).
Chemistry and properties
Aspartame has the chemical formula C14H18N2O5. Aspartame is the methyl ester of the dipeptide of the natural amino acids L-aspartic acid and L-phenylalanine. It is composed only of the L-isomers. It is odorless and dissolves in water. Under strongly acidic or alkaline conditions, aspartame first generates methanol by hydrolysis. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids. It is a nonpolar molecule (Ager et al. 1998).
In the European Union, it is also known under the E number (additive code) E951.
Aspartame is an attractive sweetener because it is approximately 200 times sweeter than sugar (sucrose) in typical concentrations, without the high energy value of sugar. While aspartame, like other peptides, has a caloric value of 4 kilocalories (17 kilojoules) per gram, the quantity of aspartame needed to produce a sweet taste is so small that its caloric contribution is negligible, which makes it a popular sweetener for those trying to avoid calories from sugar.
The taste of aspartame is not identical to that of sugar: the sweetness of aspartame has a slower onset and longer duration than that of sucrose, and some consumers find it unappealing. Blends of aspartame with acesulfame potassium are purported to have a more sugar-like taste, and to be more potent than either sweetener used alone.
Like many other peptides, aspartame may hydrolyze (break down) into its constituent amino acids under conditions of elevated temperature or high pH. This makes aspartame undesirable as a baking sweetener, and prone to degradation in products hosting a high-pH, as required for a long shelf life. The stability of aspartame under heating can be improved to some extent by encasing it in fats or in maltodextrin. The stability when dissolved in water depends markedly on pH. At room temperature, it is most stable at pH 4.3, where its half-life is nearly 300 days. At pH 7, however, its half-life is only a few days. Most soft-drinks have a pH between 3 and 5, where aspartame is reasonably stable. In products that may require a longer shelf life, such as syrups for fountain beverages, aspartame is sometimes blended with a more stable sweetener, such as saccharin.
In products such as powdered beverages, the amine in aspartame can undergo a Maillard reaction with the aldehyde groups present in certain aroma compounds. The ensuing loss of both flavor and sweetness can be prevented by protecting the aldehyde as an acetal.
Discovery and approval
Aspartame was discovered in 1965 by James M. Schlatter, a chemist working for G. D. Searle & Company. Schlatter had synthesized aspartame in the course of producing an anti-ulcer drug candidate. He discovered its sweet taste serendipitously when, in preparation to picking up a piece of paper, he licked his finger, which had accidentally become contaminated with aspartame (Blachford 2002).
Following initial safety testing, there was debate as to whether these tests had indicated that aspartame may cause cancer in rats; as a result, the U.S. Food and Drug Administration (FDA) did not approve its use as a food additive in the United States for many years.
Aspartame was approved for use in dry foods in 1974, but Searle was not allowed to market it until 1981 (GAO 1987). In early 1980, the FDA convened a Public Board of Inquiry (PBOI) consisting of three scientists charged with examining the purported relationship between aspartame and brain cancer. The PBOI concluded that aspartame does not cause brain damage, but it recommended against approving aspartame at that time, citing unanswered questions about cancer in laboratory rats. Under its authority, it also revoked approval for aspartame to be part of dry foods until more testing was done. However, on July 18, 1981, the FDA Commissioner, Arthur Hull Hayes, overturned the board decision and approved aspartame's use in dry foods (GAO 1987). Among other reasons, he cited a Japanese study that had not been available to the board. In 1983, the FDA further approved aspartame for use in carbonated beverages. In 1985, the American Medical Association further supported the conclusion of the FDA that aspartame was safe (Blachford 2002). In 1993, aspartame was approved for use in other beverages, baked goods, and confections. In 1996, the FDA removed all restrictions from aspartame allowing it to be used in all foods.
In 1985, G.D. Searle was purchased by Monsanto. In this acquisition, Searle’s aspartame business became a separate Monsanto subsidiary, the NutraSweet Company. Monsanto subsequently sold the NutraSweet company to J.W. Childs Equity Partners II L.P. on May 25, 2000. The U.S. patent on aspartame expired in 1992, and the aspartame market is now hotly contested between the NutraSweet Company and other manufacturers, such as Ajinomoto and Merisant. Another manufacturer, the Holland Sweetener Company, left the business in 2006 due to a "persistently unprofitable business position" because "global aspartame markets are facing structural oversupply, which has caused worldwide strong price erosion over the last 5 years" (DSM 2006).
Metabolism
Upon ingestion, aspartame breaks down into several residual chemicals, including aspartic acid, phenylalanine, and methanol, as well as formaldehyde (Trocho et al. 1998) and formic acid. There is some controversy surrounding the rate of breakdown into these various products and the effects that they have on those that consume aspartame-sweetened foods.
The naturally-occurring essential amino acid phenylalanine is a health hazard to those born with phenylketonuria (PKU), a rare inherited disease that prevents the essential amino acid phenylalanine from being properly converted into tyrosine and eventually being metabolized. Since individuals with PKU must consider aspartame as an additional source of phenylalanine, aspartame-containing foods sold in the United States must state "Phenylketonurics: Contains Phenylalanine" on their product labels.
Aspartame controversy
Aspartame has been the subject of controversy regarding its safety and the circumstances of its approval by the American FDA and European FSA.
Chamberlin and Narins (2005) note that individuals and special interest groups claim aspartame damages the nervous system and that some scientists consider aspartame to be a neurotoxin, thus placing the general population at risk for neurological damage. Alleged harmful effects include seizures and change in level of dopamine (brain neurotransmitter), and systems such as lupus, multiple sclerosis, and Alzheimer's disease. Some studies have recommended further investigation into possible connections between aspartame and negative effects such as headaches, brain tumors, brain lesions, and lymphoma (Olney et al. 1996; Soffritti et al. 2006; Roberts 1991).
There are also claims of possible conflict of interest in the approval process (GAO 1986; Gordon 1987).
However, Chamberlin and Narins (2005) also note that the association of aspartame with neurological disorders is not proven and symptoms directly attributed to aspartame have not been conclusively identified.
The debate over possible adverse health effects has focused mainly on four chemical components of aspartame.
- Methanol and formaldehyde. Approximately 10% of aspartame (by mass) is broken down into methanol in the small intestine. Most of the methanol is absorbed and quickly converted into formaldehyde. In high concentration, formaldehyde can kill cells and tissues, and formaldehyde can be converted to formic acid, that can cause metabolic acidosis (Chamberlin and Narins 2005). Some experts/scientists believe that the metabolism of aspartame does not damage the body because: (a) the quantity of methanol produced is too small to disrupt normal physiological processes; (b) methanol and formaldehyde are natural by-products of human metabolism and are safely processed by various enzymes; and (c) there is more methanol in some natural fruit juices and alcoholic beverages than is derived from aspartame ingestion (Lajtha et al. 1994). Other experts/scientists believe that (a) fruit juices and alcoholic beverages contain protective chemicals such as ethanol that block conversion of methanol into formaldehyde, while beverages with aspartame contain no "protective factors"; (b) exposure to very low levels of methanol and formaldehyde have been proven to cause chronic toxicity in humans; and (c) the low levels of methanol and formaldehyde in natural human metabolism are tightly-controlled and small increases above these levels can contribute to chronic poisoning (Monte 1984).
- Phenylalanine. One of the functional groups in aspartame is phenylalanine, which is unsafe for those born with phenylketonuria, a rare genetic condition. Phenylalanine is an amino acid commonly found in foods. Approximately 50 percent of aspartame (by mass) is broken down into phenylalanine, which is completely safe for everyone except sufferers of phenylketonuria. Because aspartame is metabolized and absorbed very quickly (unlike phenylalanine-containing proteins in foods), it is known that aspartame could spike blood plasma levels of phenylalanine (Stegink et al. 1987). The debate centers on whether a significant spike in blood plasma phenylalanine occurs at typical aspartame ingestion levels, whether a sudden influx of phenylalanine into the bloodstream adversely affects uptake of other amino acids into the brain and the production of neurotransmitters (since phenylalanine competes with other Large Neutral Amino Acids (LNAAs) for entry into the brain at the blood brain barrier), and whether a significant rise in phenylalanine levels would be concentrated in the brain of fetuses and be potentially neurotoxic.
- Aspartic acid. Food contains aspartic acid as an amino acid bound to proteins. Approximately 40 percent of aspartame (by mass) is broken down into aspartic acid. Because aspartame is metabolized and absorbed very quickly (unlike aspartic acid-containing proteins in foods), it is known that aspartame can spike blood plasma levels of aspartate to very high levels. Large spikes in blood plasma aspartate levels have not been seen when ingesting natural foods. Aspartic acid belongs to a class of chemicals that in high concentrations act as an excitotoxin, inflicting damage on brain and nerve cells. High levels of excitotoxins have been shown in hundreds of animal studies to cause damage to areas of the brain unprotected by the blood-brain barrier and a variety of chronic diseases arising out of this neurotoxicity. The debate is complex and has focused on several areas: (a) whether the increase in plasma aspartate levels from typical ingestion levels of aspartame is enough to cause neurotoxicity in one dose or over time, (b) whether humans are susceptible to the neurotoxicity from aspartic acid seen in some animal experiments, (c) whether aspartic acid increases the toxicity of formaldehyde, (d) whether neurotoxicity from excitotoxins should consider the combined effect of aspartic acid and other excitotoxins such as glutamic acid from monosodium glutamate.
- Aspartylphenylalanine diketopiperazine This type of diketopiperazine (DKP) is created in products as aspartame breaks down over time. Concern among some scientists has been expressed that this form of DKP would undergo a nitrosation process in the stomach producing a type of chemical that could cause brain tumors. Other scientists think that the nitrosation of aspartame or the DKP in the stomach would not produce a chemical that would cause brain tumors. In addition, only a minuscule amount of the nitrosated chemical would be produced.
ReferencesISBN links support NWE through referral fees
- Ager, D. J., D. P. Pantaleone, S. A. Henderson, A. R. Katritzky, I. Prakash, and D. E. Walters. 1998. Commercial, synthetic nonnutritive sweeteners. Angewandte Chemie International Edition 37(13): 1802-1817.
- Blachford, S. L. (Ed.). 2002. Aspartame. Thomas Gale. In eNotes.com. 2006. Retrieved May 24, 2007.
- Chamberlin, S. L., and B. Narins. 2005. The Gale Encyclopedia of Neurological Disorders. Detroit: Thomson Gale. ISBN 078769150X.
- DSM. 2006. Holland sweetener company to exit from aspartame business. Market Wire March 30, 2006. Retrieved May 24, 2007.
- General Accounting Office (GAO). 1986. Report to the Honorable Howard M. Metzenbaum, U. S. Senator: Six former HHS employees' involvement in aspartame's approval. GAO/HRD-86-109BR. Retrieved May 24, 2007.
- General Accounting Office (GAO). 1987. Report to the Honorable Howard M. Metzenbaum, U.S. Senator: Federal Department of Agriculture, Food additive approval process followed for aspartame. GAP/HRD-87-46 June 1987. Retrieved May 24, 2007.
- Gordon, G. 1987. NutraSweet: Questions Swirl. United Press International. Retrieved May 24, 2007.
- Herbst, S. T. 2001. The New Food Lover's Companion: Comprehensive Definitions of Nearly 6,000 Food, Drink, and Culinary Terms. Barron's Cooking Guide. Hauppauge, NY: Barron's Educational Series. ISBN 0764112589.
- Lajtha, A., M. A. Reilly, and D. S. Dunlop. 1994. Aspartame consumption: Lack of effects on neural function. The Journal of Nutritional Biochemistry 5(6): 266-283.
- Møller, S. 1991. Effect of aspartame and protein, administered in phenylalanine-equivalent doses, on plasma neutral amino acids, aspartate, insulin and glucose in man. Pharmacol Toxicol 68(5): 408-412.
- Monte, W. C. 1984. http://www.dorway.com/monte84.html Aspartame: Methanol and the public health]. Journal of Applied Nutrition 36(1). Retrieved May 24, 2007.
- Olney, J. W., N.B. Farber, E. Spitznagel, and L. N. Robins. 1996. Increasing brain tumor rates: Is there a link to aspartame? Journal of Neuropathology and Experimental Neurology 55: 1115-1123.
- Roberts, H. J. 1991. Does aspartame cause human brain cancer. Journal of Advancement in Medicine 4(4): 231-241.
- Soffritti, M. et al. 2006. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environmental Health Perspectives 114(3): 379-385.
- Stegink, L., L. Filer, E. Bell, and E. Ziegler. 1987. Plasma amino acid concentrations in normal adults administered aspartame in capsules or solution: Lack of bioequivalence. Metabolism 36(5): 507-512.
- Trocho, C., R. Pardo, I. Rafecas, J. Virgili, X. Remesar, J. A. Fernandez-Lopez, and M. Alemany. 1998. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 63(5): 337-349.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
