Ozone
| Ozone | |
|---|---|
| |
| General | |
| Systematic name | Trioxygen |
| Molecular formula | O3 |
| Molar mass | 47.998 g/mol |
| Appearance | bluish colored gas |
| CAS number | [10028-15-6] |
| Properties | |
| Density and phase | 2.144 g/l (0 °C), gas |
| Solubility in water | 0.105 g/100 ml (0 °C) |
| Melting point | 75.95 K, −197.2 °C |
| Boiling point | 161.25 K, −111.9 °C |
| Thermodynamic data | |
| Standard enthalpy of formation ΔfH°solid |
+142.3 kJ/mol |
| Standard molar entropy S°solid |
237.7 J.K−1.mol−1 |
| Hazards | |
| EU classification | not listed |
| NFPA 704 | |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Regulatory data | Flash point, RTECS number, etc. |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
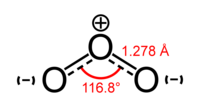
Ozone (O3) is a triatomic molecule consisting of three oxygen atoms. It is an allotrope of oxygen (O2), a much more stable diatomic molecule.
Ozone is a pale blue gas at standard temperature and pressure. It forms a dark blue liquid below −112 °C and a dark blue solid below −193 °C. Ozone is a powerful oxidizing agent. It is also unstable, decaying to ordinary oxygen through the reaction:
- 2 O3 → 3 O2
This reaction proceeds more rapidly with increasing temperature and decreasing pressure.
It is present in low concentrations throughout the Earth's atmosphere: ground level ozone is an air pollutant with harmful effects on lung function and in the upper atmosphere it prevents damaging ultraviolet light from reaching the Earth's surface. It is also formed from O2 by electrical discharges such as lightning, and by action of high energy electromagnetic radiation.
Some kinds of electrical equipment generate significant levels of ozone. This is especially true of devices using high voltages, such as television sets, laser printers, and photocopiers. Electric motors using brushes can generate ozone from repeated sparking inside the unit. Large motors, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors.
Discovery of ozone
Ozone was discovered by Christian Friedrich Schönbein in 1840, who named it after the Greek word for smell (ozein), from the peculiar odor in lightning storms. [1]. The odor from a lightning strike is from electrons freed during the rapid chemical changes, not the ozone itself [2]. Sax's Dangerous Properties of Industrial Materials, 8th. ed. indicates that ozone is colorless (perhaps pale blue) in gas but dark blue as a liquid. In concentrations of 0.015ppm, ozone has a barely detectable odor. At 1 ppm it has a sulfur-like odor. However the smell is also attributed to the discharge of the electrical current from the dipole bonding and reformation of the oxygen model, which at high temperatures is known to release an inert odor.
Ozone in Earth's atmosphere
Ozone layer
See main article: Ozone layer.
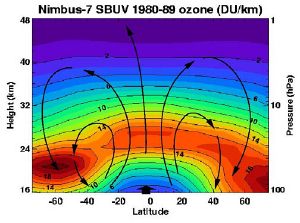
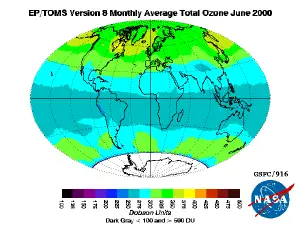
The highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer. Here it filters out the shorter wavelengths (less than 320 nm) of ultraviolet light (270 to 400 nm) from the Sun that would be harmful to most forms of life in large doses. These same wavelengths are also responsible for the production of vitamin D, which is essential for human health. The standard way to express total ozone amounts in the atmosphere is by using Dobson units. Ozone used in industry is measured in ppm (OSHA exposure limits for example), and percent by mass or weight.
Air pollution
See main articles: Tropospheric ozone and Air pollution.
Ozone is not directly emitted by car engines or by industrial operations themselves. These sources emit hydrocarbons and nitrogen oxides that react with sunlight to form ozone directly at the source of the pollution being emitted and in the atmosphere's boundary layer (1 to 3 km altitude). The mix of hydrocarbons, nitrogen oxides, and ozone are the major components of smog that frequently occurs in urban and suburban areas. Recent satellite maps of nitrogen dioxide (NO2) clearly show the worldwide distribution of polluted regions associated with industrial activity (automobiles, factories, and fossil fuel power generation).
There is a great deal of evidence to show that ozone at the earth's surface can harm lung function and irritate the respiratory system (WHO Europe reports, cited below). Ozone has been found to convert cholesterol in the blood stream to plaque (which causes hardening and narrowing of arteries). This cholesterol product has also been implicated in Alzheimer's disease, suggesting a link between the inflammatory response associated with head injury and Alzheimer's. Air quality guidelines such as those from the World Health Organization are based on detailed studies of what levels can cause measurable health effects.
There is also evidence of significant reduction in agricultural yields due to increased ground-level ozone which interferes with photosynthesis and stunts overall growth of some plant species [3][4].
Although ozone was present at ground level before the industrial revolution, peak concentrations are far higher than the pre-industrial levels [5] and even background concentrations well away from sources of pollution are substantially higher [6].
Ozone reacts directly with some hydrocarbons such as aldehydes and thus begins their removal from the air, but the products of ozonolysis are themselves key components of smog. Ozone photolysis by UV light leads to production of the hydroxyl radical and this plays a part in the removal of hydrocarbons from the air, but is again a step in the creation of components of smog such as peroxyacyl nitrates which are powerful eye irritants. Ultimately, ozone is one component of smog which is harmful in itself and contributes both to the production and ultimate removal of other air pollutants.
Industrial production
Industrially, ozone is produced with short wavelength ultraviolet radiation from a mercury vapor lamp or the application of a high voltage electrical field in a process called cold or corona discharge. The cold discharge apparatus consists of two metal plates separated by an air gap and a high dielectric strength electrical insulator such as borosilicate glass or mica. A high voltage alternating current is applied to the plates and the ozone is formed in the air gap when O2 molecules disassociate and recombine into O3. A faint corona may be present in the air gap, but the voltage is maintained below that which would cause punch-through of the insulator with subsequent arcing and plasma formation. In the laboratory ozone can be produced by electrolysis using a 9 volt battery, a pencil graphite rod cathode, a platinum wire anode and a 3M sulfuric acid electrolyte [1]. The half cell reactions taking place are:
So that in the net reaction three equivalents of water are converted into one equivalent of ozone and one equivalent of hydrogen. Oxygen formation is a competing reaction...
Reactions
Ozone will oxidize metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state:
- 2 Co2+ + 2 H+ + O3 → 2 Co3+ + H2O + O2
Ozone oxidizes oxides to peroxides. It also oxidizes oxides to oxides of higher oxidation number:
- SO2 + O3 → SO3 + O2
- NO + O3 → NO2 + O2
The above reaction is accompanied by chemiluminescence. The NO2 can be further oxidized:
- NO2 + O3 → NO3 + O2
The NO3 formed can react with NO2 to form N2O5:
- NO2 + NO3 → N2O5
Ozone reacts with carbon to form carbon dioxide, even at room temperature:
- C + 2 O3 → CO2 + 2 O2
Ozone does not react with ammonium salts but it reacts with ammonia to form ammonium nitrate:
- NH3 + 4 O3 → NH4NO3 + 4 O3 + H20
Ozone reacts with sulfides to make sulfates:
- PbS + 4 O3 → PbSO4 + 4 O2
Sulfuric acid can be produced from ozone, either starting from elemental sulfur or from sulfur dioxide:
- S + H2O + O3 → H2SO4
- 3 SO2 + 3 H2O + O3 → 3 H2SO4
All three atoms of ozone may also react, as in the reaction with tin(II) chloride and hydrochloric acid:
- 3 SnCl2 + 6 HCl + O3 → 3 SnCl4 + 3 H2O
In the gas phase, ozone reats with hydrogen sulfide to form sulfur dioxide:
- H2S + O3 → SO2 + H2O
In an aqueous solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce sulfuric acid:
- H2S + O3 → S + O2 + H2O
- 3 H2S + 4 O3 → 3 H2SO4
Iodine perchlorate can be made by treating iodine dissolved in cold anhydrous perchloric acid with ozone:
- I2 + 6 HClO4 + O3 → 2 I(ClO4)3 + 3 H2O
Solid nitryl perchlorate can be made from NO2, ClO2, and O3 gases:
- 2 NO2 + 2 ClO2 2 O3 → 2 NO2ClO4 + O2
Ozone can be used for combustion reactions and combusting gases in ozone provides higher temperatures than combusting in dioxygen (O2). Following is a reaction for the combustion of carbon subnitride:
- 3 C4N2 + 4 O3 → 12 CO + 3 N2
Ozone can react at cryogenic temperatures. At 77 K (-196 °C), atomic hydrogen reacts with liquid ozone to form a hydrogen superoxide radical, which dimerizes[2]:
- H + O3 → HO2 + O
- 2 HO2 → H2O4
Ozonides can be formed, which contain the ozonide anion, O3-. These compounds are explosive and must be stored at cryogenic temperatures. Ozonides for all the alkali metals are known. KO3, RbO3, and CsO3 can be prepared from their respective superoxides:
- KO2 + O3 → KO3 + O2
Although KO3 can be formed as above, it can also be formed from [[potassium hydroxide]] and ozone[3]:
- 2 KOH + 5 O3 → 2 KO3 + 5 O2 + H2O
NaO3 and LiO3 must be prepared by action of CsO3 in liquid NH3 on an ion exchange resin containing Na+ or Li+ ions[4]:
- CsO3 + Na+ → Cs+ + NaO3
Treatment with ozone of calcium dissolved in ammonia leads to ammonium ozonide and not calcium ozonide[5]:
- 3 Ca + 10 NH3 + 6 O3 → Ca•6NH3 + Ca(OH)2 + Ca(NO3)2 + 2 NH4O3 + 2 O2 + H2
Ozone can be used to remove manganese from the water, forming a precipitate which can be filtered:
- 2 Mn2+ + 2 O3 + 4 H2O → 2 MnO(OH)2 (s) + 2 O2 + 4 H+
Ozone will also turn cyanides to the one thousand times less toxic cyanates:
- CN- + O3 → CNO- + O2
Finally, ozone will also completely decompose urea[6]:
- (NH2)2CO + O3 → N2 + CO2 + 2 H2O
Use in industry
Ozone can be used for bleaching substances and for killing bacteria. Many municipal drinking water systems kill bacteria with ozone instead of the more common chlorine. Ozone does not form organochlorine compounds, but it also does not remain in the water after treatment, so some systems introduce a small amount of chlorine to prevent bacterial growth in the pipes, or may use chlorine intermittently, based on results of periodic testing. Where electrical power is abundant, ozone is a cost-effective method of treating water, as it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odor in drinking water.
Industrially, ozone or ozonated water is used to:
- disinfect water before it is bottled,
- kill bacteria on food-contact surfaces
- scrub yeast and mold spores from the air in food processing plants
- wash fresh fruits and vegetables to kill yeast, mold and bacteria
- chemically attack contaminants in water (iron, arsenic, hydrogen sulfide, nitrites, and complex organics lumped together as "color"),
- provide an aid to flocculation (a process of agglomeration of molecules, which aids in filtration... this is where the iron and arsenic are removed),
- clean and bleach fabrics (the latter use is patented),
- assist in processing plastics to allow adhesion of inks,
- age rubber samples to determine the useful life of a batch of rubber.
Ozone is a reagent in many organic reactions in the laboratory and in industry. Ozonolysis is the cleavage of an alkene to carbonyl compounds.
Use in medicine
Ozone, along with hypochlorite ions, is naturally produced by white blood cells and the roots of marigolds as a means of destroying foreign bodies. When ozone breaks down it gives rise to oxygen free radicals, which are highly reactive and damage or destroy most organic molecules.
Ozone has a number of medical uses. It can be used to affect the body's antioxidant-prooxidant balance, since the body usually reacts to its presence by producing antioxidant enzymes. Many hospitals in the U.S. and around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned and then sealed airtight before being filled with ozone which effectively kills or neutralizes all remaining bacteria.
Ozone therapy has blossomed into a thriving field of alternative medicine, and there are a host of claimed applications above and beyond what has actually been verified by studies.
In the United States ozone therapy is illegal, as the Food and Drug Administration (FDA) has not approved its use on humans. Medical ozone therapy is recognized in Bulgaria, Cuba, Czech Republic, France, Germany, Israel, Italy, Mexico, Romania and Russia. It is currently used legally in 16 Nations. At least 12 states in the USA (AK, AZ, CO, GA, MN, NY, NC, OH, OK, OR, SC and WA) have passed legislation to ensure that alternative therapies are available to consumers. Physicians in those states can legally use ozone as an alternative treatment in their practice without fear of prosecution.
At least one death has been attributed to application of ozone through insufflation in the U.S. "Air cleaners" which produce "activated oxygen", i.e., ozone, are often sold in the U.S. nonetheless. See Air ioniser.
Other uses
During the 1992 U.S. Presidential election, George H.W. Bush referred to his opponents Bill Clinton and Al Gore as "Bozo and Ozone", respectively, the latter in connection with Gore's well known stance on environmental issues.
Ozone is also popularly used in spas or hot tubs instead of Chlorine or Bromine for keeping the water free of bacteria. Ozone gas is created by an ultraviolet light bulb or corona discharge chip and injected into the plumbing system.
See also
- Ozone depletion, including the phenomenon known as the Ozone Hole.
- Ozone layer
- Tropospheric ozone
Further reading
- Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0-471-17816-0
- Greenwood, N. N.; & Earnshaw, A. 1997. Chemistry of the Elements (2nd Edn.). Oxford, UK: Butterworth-Heinemann. ISBN 0-7506-3365-4.
ReferencesISBN links support NWE through referral fees
- ↑ Laboratory Experiments on the Electrochemical Remediation of the Environment. Part 7: Microscale Production of Ozone Jorge G. Ibanez, Rodrigo Mayen-Mondragon, and M. T. Moran-Moran J. Chem. Ed. October 2005 Vol. 82 No. 10 p. 1546 Abstract
- ↑ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 44-49
- ↑ Housecroft & Sharpe, 2005. "Inorganic Chemistry." pg 439
- ↑ Housecroft & Sharpe, 2005. "Inorganic Chemistry." pg 265
- ↑ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 44-49
- ↑ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 259, 269-270
External links
- NASA's Earth Observatory article on Ozone
- International Day for the Preservation of the Ozone Layer
- International Chemical Safety Card 0068
- NIOSH Pocket Guide to Chemical Hazards
- National Institute of Environmental Health Sciences Ozone Alerts
- WHO-Europe reports: Health Aspects of Air Pollution (2003) (PDF) and "Answer to follow-up questions from CAFE (2004) (PDF)
- Ground-level Ozone Air Pollution — A summary for non specialists by GreenFacts of the above WHO reports.
- NASA Study Links "Smog" to Arctic Warming— NASA Goddard Institute for Space Studies (GISS) study shows the warming effect of ozone in the Arctic during winter and spring.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.