Ammonium nitrate
| Ammonium nitrate | |
|---|---|
| |
| General | |
| Systematic name | Ammonium nitrate |
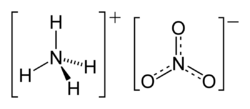
| Molecular formula | NH4NO3 |
| Molar mass | 80.04336 g/mol |
| Appearance | white solid |
| CAS number | [6484-52-2] |
| Properties | |
| Density and phase | 1.73 g/cm³, solid |
| Solubility in water | 119 g/100 ml (0 °C) 190 g/100 ml (20 °C) 286 g/100 ml (40 °C) 421 g/100 ml (60 °C) 630 g/100 ml (80 °C) 1024 g/100 ml (100 °C) |
| Melting point | 169 °C |
| Boiling point | approx. 210 °C decomp |
| Detonation velocity | 5,270 m/s |
| Critical relative humidity | 78% (0 °C) 65% (20 °C) 58.5% (30 °C) 52.5% (40 °C) 46.5% (50 °C) 41% (60 °C) |
| Nitrogen content | 34.5%N |
| Structure | |
| Coordination geometry |
? |
| Crystal structure | trigonal |
| Hazards | |
| MSDS | External MSDS |
| EU classification | not listed |
| NFPA 704 | |
| RTECS number | BR9050000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Ammonium nitrite Ammonium perchlorate |
| Other cations | Sodium nitrate Potassium nitrate Hydroxylammonium nitrate |
| Related compounds | Nitrous oxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
The chemical compound ammonium nitrate, the nitrate of ammonia with the chemical formula NH4NO3, is a white powder at room temperature and standard pressure. It is commonly used in agriculture as a high-nitrogen fertilizer, and it has also been used as an oxidizing agent in explosives, especially improvised explosive devices.
Production
Industrial production is chemically quite simple, although technologically challenging. The acid-base reaction of ammonia with nitric acid gives a solution of ammonium nitrate: HNO3(aq) + NH3(g) → NH4NO3(aq). For industrial production, this is done using anhydrous ammonia gas and concentrated nitric acid. This reaction is violent and very exothermic. It should never be attempted by amateurs or in improvised equipment using such concentrated materials, though with plenty of dilution by water, it could be considered easy. After the solution is formed, typically at about 83 percent concentration, the excess water is evaporated to an ammonium nitrate (AN) content of 95 to 99.9 percent concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce. The processes involved are simple in principle, but certainly not easy.
The Haber process combines nitrogen and hydrogen to produce ammonia, part of which can be oxidized to nitric acid and combined with the remaining ammonia to produce the nitrate. Another production method is used in the so-called Odda process.
Crystalline phases
Transformations of the crystal states due to changing conditions (temperature, pressure) affect the physical properties of ammonium nitrate. The following crystalline states have been identified [1]:
| System | Temperature (°C) | State | Volume Change (%) |
|---|---|---|---|
| - | >169.6 | liquid | - |
| I | 169.6 to 125.2 | cubic | +2.1 |
| II | 125.5 to 84.2 | tetragonal | -1.3 |
| III | 84.2 to 32.3 | α-rhombic | +3.6 |
| IV | 32.3 to −16.8 | β-rhombic | −2.9 |
| V | −16.8 | tetragonal | - |
Other uses

The most common use of ammonium nitrate is in fertilizers. This is due to its high nitrogen content—a desirable feature for fertilizers, as plants require nitrogen to make proteins—and inexpensive industrial manufacture.
Ammonium nitrate is also used in instant cold packs. In this use, ammonium nitrate is mixed with water in an endothermic reaction, which absorbs 26.2 kilojoules of heat per mole of reactant.
Products of Ammonium nitrate reactions are used in Airbags. Sodium azide (NaN3) is the chemical used in airbags, as it decomposes to Na (s) and N2 (g).
Ammonium nitrate is used in the treatment of some titanium ores.
Ammonium nitrate is used in the preparation of nitrous oxide (N2O):
- NH4NO3(aq) -> N2O(g) + 2H2O(l)
Ammonium nitrate is used in survival kits mixed with zinc dust and ammonium chloride because it will ignite on contact with water.
Ammonium nitrate can be used to make anhydrous ammonia, a chemical often used in the production of methamphetamine.
Use in explosives
As a strong oxidizing agent, ammonium nitrate makes an explosive mixture when combined with a fuel such as a hydrocarbon, usually diesel fuel (oil) or, sometimes, kerosene. Because ammonium nitrate and fuel oil (ANFO) are readily available in bulk, ANFO mixtures have occasionally been used for improvised bombs—for example by the Provisional IRA and in the Oklahoma City bombing.
Ammonium nitrate is used in military explosives such as the daisy cutter bomb, and as a component of amatol. Military mixtures are often spiked with about 20 percent aluminum powder as well, increasing the blast power, but with some loss of brisance. One example of this is Ammonal, which contains ammonium nitrate, TNT & aluminum. Aluminized mixtures are very effective under confinement, as in underwater demolition, torpedoes, and rock blasting. Very cheap water-based blasting slurries tap the power of an aluminum-water reaction with enough ammonium nitrate added to burn off the resulting hydrogen.
Ammonium nitrate is also an explosive in its purest form although it is an unusually insensitive one. Explosive properties become much more evident at elevated temperatures. When ammonium nitrate is fused and "boiled" to generate nitrous oxide, it has been claimed to be as sensitive as dynamite at about the 240 °C operating temperature.
This exothermic reaction can run away and reach detonation velocities (without proper temperature controls). The extent of this possibility has been demonstrated several times, most notably at the Ohio Chemical plant in Montreal in 1966. Millions of pounds of relatively pure ammonium nitrate have been (accidentally) detonated when subjected to severe heat and/or shocks (see "Disasters" below). Ammonium nitrate has also found use as a solid rocket propellant, but for a while ammonium perchlorate was frequently considered preferable due to higher performance and faster burn rates. Lately, favor has been swinging back towards ammonium nitrate in rocketry, as it delivers almost as much thrust without producing an exhaust jet full of gaseous hydrochloric acid (HCl) and without the extra expense and sensitivity hazards. Fertilizer-grade ammonium nitrate (FGAN) is manufactured in more compact form, with much lower porosity, in order to achieve greater stability and lower sensitivity to detonation, whereas technical grade ammonium nitrate (TGAN) prills are made to be porous for better absorption of fuel and higher reactivity.
Disasters
Ammonium nitrate decomposes into gases including oxygen when heated (non-explosive reaction); however, ammonium nitrate can be induced to decompose explosively by detonation. Large stockpiles of the material can be a major fire risk due to their supporting oxidation, and may also detonate, as happened in the Texas City disaster of 1947, which led to major changes in regulations for storage and handling.
There are two main types of incidents that result in explosions:
- The explosion happens by a mechanism known as "shock to detonation transition." It may be initiated by an explosive charge going off in the mass, or the detonation of a shell thrown into the mass, or the detonation of an explosive mixture in contact with the mass. (See the examples of incidents at Oppau and Tessenderlo, mentioned below.)
- The explosion results from a fire that spreads into the ammonium nitrate itself, or to a mixture of ammonium nitrate with a combustible material during the fire. (See the examples of incidents at Texas City and Brest, noted below.) The fire must be confined at least to a degree, for transition from a fire to an explosion (a phenomenon known as "transition from a decomposition or deflagration," or DDT).
Pure, compact ammonium nitrate is stable, but it decomposes at temperatures above 210 °C. It stops decomposing once the heat source is removed, but when catalysts are present (including combustible materials, acids, metal ions, or chlorides), the reaction can become self-sustaining (known as "self-sustaining decomposition," SSD). This is a well-known hazard with some types of NPK fertilizers and is responsible for the loss of several cargo ships.
Some examples of disasters involving ammonium nitrate are given below.
- Oppau, Germany, 1921: An attempt to disaggregate a fertilizer mix using industrial explosives caused the death of 450 people and the destruction of 700 houses on September 21, 1921. The fertilizer was a 50:50 mixture of ammonium nitrate and ammonium sulphate. It was claimed that the factory had used this method of disaggregation over 20,000 times without incident. It is thought that on this occasion, poor mixing had led to certain parts of the mass to contain more ammonium nitrate than others. Of the 4500 tonnes of fertilizer stored in the warehouse, only one-tenth exploded.
- Tessenderlo, Belgium, 1942: Another attempt to disaggregate a pile of 150 tonnes of ammonium nitrate with industrial explosives ended tragically on April 29, 1942. Several hundred people were killed.
- Texas City, United States, 1947: The cargo ship Grandcamp was being loaded on April 16, 1947, when a fire was detected in the hold—at this point, 2600 tonnes of ammonium nitrate in sacks was already aboard. The captain responded by closing the hold and pumping in pressurized steam. One hour later, the ship exploded, killing several hundred people and setting fire to another vessel, the High Flyer, which was moored 250 metres away and which contained 1050 tonnes of sulfur and 960 metric tons of ammonium nitrate. The Grandcamp explosion also created a powerful earthshock and knocked two small planes flying at 1500 feet out of the sky. The High Flyer exploded the next day, after having burned for sixteen hours. 500 tonnes of ammonium nitrate on the quayside also burned, but without exploding, probably due to the fact that it was less tightly packed.
- Brest, France, 1947: The cargo ship Ocean Liberty was loaded with 3300 tonnes of ammonium nitrate and various inflammable products when it caught fire at 12:30, on July 28, 1947. The captain ordered the hold to be sealed and pressurised steam was pumped in. As this did not stop the fire, the vessel was towed out of the harbour at 14:00, and exploded at 17:00. The explosion caused 29 deaths and serious damage to the port of Brest.
- Roseburg, Oregon, 1959: A truck carrying dynamite and ammonium nitrate caught fire early in the morning of August 7, 1959. When it exploded, it killed 14 people and injured 125 more. Several blocks of downtown Roseburg were destroyed. The accident is locally referred to as "The Blast."
- Kansas City, Missouri, 1988: On November 29, 1988, at 4:07 AM two trailers containing approximately 50,000 lbs of ammonium nitrate exploded at a construction site located near the 87th street exit of Highway 71 in Kansas City, Missouri. The explosives were to be used in the blasting of rock while constructing Highway 71. The explosions resulted in the deaths of six firemen from the Kansas City Fire Department's Pumper Companies 30 and 41. The blasts created two craters (each approximately 100 feet wide and eight feet deep), shattered windows within a 10-mile area, and could be heard 40 miles away. It was later determined that the explosions were acts of arson, set by individuals embroiled in a labor dispute with the construction company contracted to build the highway.
- Toulouse, France, 2001: On September 21, 2001, at 10:15 AM, in the AZF (Azote de France) fertilizer factory in Toulouse, France, an explosion occurred in a warehouse where the off-specification granular AN was stored flat, separated by partitions. About 200 to 300 tons is said to be involved in the explosion, resulting in 31 people dead and 2,442 injured, 34 of them seriously. The blast wave shattered windows up to three kilometers away and the resulting crater was ten meters deep and 50 meters wide. The exact cause remains unknown. The material damage was estimated at 2.3 billion euros.[2]
- Ryongchon, North Korea, 2004: A freight train carrying ammonium nitrate exploded in this important railway town near the Chinese border on April 22, 2004, killing 162 people and injuring over 3,000 others. The station was destroyed, as were most buildings within 500 meters, and nearly 8,000 homes were destroyed or damaged. Two craters of about ten meters in depth were seen at the site of the explosion.
- Beirut, Lebanon, 2020: On August 4, 2020, a large amount of ammonium nitrate stored at the port in Beirut, the capital of Lebanon, exploded, causing at least 207 deaths, 7,500 injuries, and US$15 billion in property damage, and leaving an estimated 300,000 people homeless. A cargo of 2,750 tonnes of the substance (equivalent to around 1.1 kilotons of TNT) had been stored in a warehouse without proper safety measures for the previous six years, after having been confiscated by the Lebanese authorities from the abandoned ship MV Rhosus. The explosion was preceded by a fire in the same warehouse.[3]
Notes
- ↑ The type V crystal form was reported by M. Herrmann, W. Engel, J. Schneider, and H. Goebel in Materials Science Forum, 1994 (166, 489). This is a quasi-cubic form that is related to cesium chloride—the nitrogen atoms of the nitrate ions and the ammonium ions are at the sites in a cubic array where Cs and Cl would be in the CsCl lattice. See C.S. Choi and H.J. Prask, Acta Crystallographica B, 1983, 39, (414-420).
- ↑ Marianne Arens and François Thull, Chemical explosion in Toulouse, France leaves at least 29 dead World Socialist Web Site, September 25, 2001. Retrieved July 21, 2021.
- ↑ Sarah el Deeb and Bassem Mroue, In a horrific instant, a burst of power that ravaged Beirut Associated Press, August 5, 2020. Retrieved July 21, 2021.
ReferencesISBN links support NWE through referral fees
- Brown, Theodore E., H. Eugene LeMay, and Bruce E. Bursten. Chemistry: The Central Science. 10th ed. Upper Saddle River, NJ: Prentice Hall, 2005. ISBN 0131096869
- Chang, Raymond. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math, 2006. ISBN 0073221031
- Housecroft, Catherine E., and Alan G. Sharpe. Inorganic Chemistry. 4th ed. Harlow, UK: Prentice Hall, 2001. ISBN 0582310806
- McMurry, John, and Robert C. Fay. Chemistry. 4th ed. Upper Saddle River, NJ: Prentice Hall, 2004. ISBN 0131402080
- Moore, John W., Conrad L. Stanitski, and Peter C. Jurs. Chemistry: The Molecular Science. New York: Harcourt College, 2002. ISBN 0030320119
- Smith, Roland. Conquering chemistry. Sydney: McGraw-Hill, 1994. ISBN 0074701460
- UNIDO and International Fertilizer Development Center. Fertilizer Manual. Kluwer Academic Publishers, 1998. ISBN 0792350324
External links
All links retrieved July 25, 2023.
- Ammonium Nitrate Security Program US Department of Homeland Security
- "Storing and Handling Ammonium Nitrate", UK Health and Safety Executive publication INDG230, 1986.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
