Methylene blue
| Methylene blue | |
|---|---|
| |
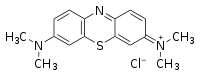
| Chemical name | 3,7-bis(Dimethylamino)- phenothiazin-5-ium chloride or Tetramethyl thionine |
| Empirical formula | C16H18ClN3S |
| Molecular mass | 319.86 g/mol |
| CAS number | [61-73-4] |
| EC number | 200-515-2 |
| Melting point | 100 ¬įC |
| Boiling point | Decomposes |
| SMILES[1] | CN(C)c3ccc2nc1ccc(N(C) C)cc1[s+]c2c3.[Cl-] |
| Disclaimer and references | |
Methylene blue (or MB) is a basic aniline dye with the molecular formula C16H18N3SCl. At room temperature, it appears as a solid, odorless, dark green powder that yields a blue solution when dissolved in water. It has many uses in a number of different fields. For instance, chemists use it to detect oxidizing agents and biologists use it to stain tissue samples and detect nucleic acids. In medicine, it is used as a treatment for various illnesses and disorders, including methemoglobinemia, schizophrenia, kidney stones, and herpes infections. In aquaculture, it is used to prevent freshwater fish eggs from being infected by bacteria and fungi.
Other Names
Trade names for methylene blue include Desmoid piller, Desmoidpillen, Panatone, Urolene Blue, and Vitableu. Its synonyms include Phenothiazine-5-ium, 3,7-bis(dimethylamino)-, chloride (9CI), C.I. Basic Blue 9 (8CI), methylthionine chloride, methylthioninium chloride, tetramethylthionine chloride, swiss blue, aizen methylene blue, and C.I. 52015. Methylene blue should not be confused with methyl blue, another histology stain, new methylene blue, nor with the methyl violets often used as pH indicators.
Uses
Chemistry
Methylene blue is widely used as a redox indicator in analytical chemistry, meaning that it indicates the presence or absence of oxygen. Oxygen-rich environments are said to be oxidizing. Some chemical elements, such as oxygen or chlorine, have such a strong attraction to electrons that they can strip electrons away from the atoms of other elements‚ÄĒthese are known as oxidizing agents. Methylene blue indicates the presence of oxidizing agents because it is oxidized by these compounds. When electrons are stripped from methylene blue, the resulting molecule imparts a blue color to the solution‚ÄĒgiving a clear sign of a chemical change.[2]
The redox properties can be seen in a classical demonstration of chemical kinetics in general chemistry, known as the "blue bottle" experiment. Typically, a solution is made of glucose, methylene blue, and sodium hydroxide. Upon shaking the bottle, the oxygen in the solution oxidizes methylene blue and the solution turns blue. The glucose will gradually reduce the methylene blue to its colorless (reduced) form. Hence, when the dissolved oxygen is entirely consumed, the solution will turn colorless.[3]
In addition, methylene blue is used to make the reaction between Fehling's solution and reducing sugars more visible. It is also a reagent in redox titrations in volumetric analysis.
Biology
Methylene blue is commonly used by biologists as a dye that assists in the identification of bacteria. Because bacteria are practically colorless, adding a drop or two of methylene blue to a microscope slide enables the biologist to see bacterial shapes and structures. A dye such as methylene blue is called a stain in biology. It works by binding to biological tissues through chemical attractions.
This dye is at its deepest shade of blue when in contact with acids. This property makes it very useful in the identification of nucleic acids, such as DNA and RNA.[4] It can also work as an alternative to the chemical crystal violet in cellular structure|gram's staining procedures.[5]
Given this attraction to nucleic acids, methylene blue has also been used to detect RNA sequences in specialized techniques such as "northern blotting" (or "northern hybridization"). In addition, methylene blue is a safer substitute for another chemical called ethidium bromide, which is often used in the visualization of DNA on gels in techniques known as "western blots".
The use of ethidium bromide has several disadvantages. It is a potent carcinogen and mutagen. In addition, the short-wavelength ultraviolet light required to detect DNA by the fluorescence of ethidium bromide can cause unwanted mutations in the DNA sample itself. The drawbacks of using methylene blue as a replacement are that it is less sensitive than ethidium bromide, and it fades rapidly after staining. Consequently, methylene blue is not an ideal replacement, although it is suitable for demonstration experiments in schools, due to its less harmful nature.[6]
Methylene blue has also been used as a way to obtain a quick estimate of the percentage of viable cells in a yeast sample. Viable yeast cells contain an enzyme that decolorizes methylene blue, whereas dead cells do not. As a result, when yeast cells are suspended in a solution containing the dye, it stains the dead cells blue, but the live cells remain unstained. It should be noted, however, that this method simply indicates whether an enzyme is present in the yeast cells, and not whether the cells are incapable of dividing. This approach, therefore, is less accurate than other methods and should be used to simply provide a rapid estimate.[7]
Medicine
Methylene blue is widely used in the medical community. It is employed as a treatment for methemoglobinemia, a disorder in which methemoglobin (oxidized hemoglobin) levels rise above their normal one percent in blood. Methemoglobin lacks the electron needed to form a bond with oxygen and is thus incapable of oxygen transport. In other words, if there is too much methemoglobin in the blood, a person can die because of lack of oxygen supply to vital tissues and organs. Given methylene blue's reducing abilities, it can reduce the excess methemoglobin to hemoglobin, thereby restoring normal methemoglobin levels.[8]
Methylene blue can also be used as a stain for surgical and medical marking (though it can cause localized tissue inflammation), and as a diagnostic agent in renal function tests and vital nerve staining. It has also been used as an antidote for cyanide poisoning, but it should be used carefully, because it may make cyanide toxicity worse by increasing the amount of cyanide in the blood.[9] Sodium nitrite is considered to be a safer and more effective antidote.
This substance has also been used as a drug for the treatment of manic-depressive psychosis (schizophrenia), infection by herpes simplex virus, chronic urolithiasis (formation of kidney and bladder stones), and glutaricaciduria (a rare hereditary metabolic disorder, that results in the accumulation of excess organic acids in the blood and urine). Methylene blue was formerly used as a urinary antiseptic, a treatment for cystitis (bladder infection) and urethritis (infection of the urethra), as well as an analgesic (painkiller) and antipyretic (fever reducer); however, more effective agents are now used. In terms of its effectiveness as an antiparasitic, methylene blue has been shown to act against malaria.
Aquaculture
Methylene blue is used in aquaculture and by tropical fish hobbyists as a bacterial and fungal infection preventative on freshwater fish eggs. It is also commonly used as an additive in a solution (or "dip") in which infected (or newly purchased) fish are immersed. In the dip, methylene blue serves to fight and kill the offending organisms, as well as increase the oxygen-carrying capacity of the fish's hemoglobin.[10]
Some hobbyists use methylene blue to treat fish infected with ich (the parasitic protozoa Ichthyophthirius multifiliis), but methylene blue is not the most effective remedy and other solutions, such as ionized copper, are instead suggested.[11] Although methylene blue is nontoxic to fish if used at the proper dosage, it is toxic to live plants and will also harm fish if placed in long-term contact with them.
Other Uses
Methylene blue has also been used as a dye for temporary hair colorants, cotton, wool, leather, and paper.
Misuse
While methylene blue has many uses in medicine, it has also been used inappropriately by pranksters, given its ability to impart a green-blue color to urine. About 75 percent of an oral dose of methylene blue is released into the urine (mostly in its reduced form - leucomethylene blue), thereby adding a blue-green hue to the urine. (Some of the remaining methylene blue is excreted via the bile). However, use of this substance in such a way is dangerous, because methylene blue is a biologically active substance, and if administered inappropriately, it can lead to a number of health complications, including gastrointestinal disturbances and dysuria.[12] Large doses of methylene blue can produce methemoglobinemia, chest pain, dyspnea, restlessness, apprehension, tremors, a sense of oppression, urinary tract irritation, as well as a mild hemolysis with moderate hyperbilirubinemia, reticulosis, and slight anemia.[13]
Notes
- ‚ÜĎ SMILES is the acronym for Simplified Molecular Input Line Entry Specification
- ‚ÜĎ American Chemistry Council, Methylene Blue, Part 2: The Chemist's Indicator. Retrieved September 27, 2007.
- ‚ÜĎ Swiss Federal Institute of Technology Zurich, Redox behavior of methylene blue (blue bottle). Retrieved September 27, 2007.
- ‚ÜĎ American Chemistry Council, Methylene Blue, Part 1: The Biologist's Dye. Retrieved September 27, 2007.
- ‚ÜĎ Wang, Nam Sun, [http://www.eng.umd.edu/~nsw/ench485/lab9b.htm Experiment Number 9B Cell Differentiation by Gram‚Äôs Stain.] Retrieved September 27, 2007.
- ‚ÜĎ Madden, Dean. Safer Stains for DNA. Retrieved September 27, 2007.
- ‚ÜĎ Painting, K, and Kirsophttp, B, A Quick Method for Estimating the Percentage of Viable Cells in a Yeast Population, Using Methylene Blue Staining. Retrieved September 27, 2007.
- ‚ÜĎ Lee, David C., Methemoglobinemia. Retrieved September 27, 2007.
- ‚ÜĎ Drugs.com, Methylene Blue (Systemic). Retrieved September 27, 2007.
- ‚ÜĎ Ellis, Terry A., Dips. Retrieved September 27, 2007.
- ‚ÜĎ Fenner, Bob, Methylene Blue, Safe, But Not Always Efficacious. Retrieved September 27, 2007.
- ‚ÜĎ Carlson, John, Re: Students asked if methylene blue put into cookies as a prank was dangerous? Retrieved September 27, 2007.
- ‚ÜĎ Medsafe, Data sheet: Methylene Blue Solution for Injection. Retrieved September 27, 2007.
ReferencesISBN links support NWE through referral fees
- Meissner, P.E., Mandi, G., Coulibaly, B., et al. ‚ÄúMethylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine.‚ÄĚ Malaria Journal 5:84. 2006.
- National Toxicology Program. Executive Summary Methylene Blue. Retrieved September 27, 2007.
- Narsapur, S.L., and Naylor, G.J. ‚ÄúMethylene blue: A possible treatment for manic depressive psychosis.‚ÄĚ J. Affect. Disord. 5(2): 155-61. 1983.
- Schirmer, H., Coulibaly, B., Stich, A., et al. ‚ÄúMethylene blue as an antimalarial agent‚ÄĒpast and future.‚ÄĚ Redox Rep. 8:272‚Äď76. 2003.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.