Difference between revisions of "Peptide" - New World Encyclopedia
| Line 46: | Line 46: | ||
==== Growth factors ==== | ==== Growth factors ==== | ||
| − | + | Polypeptide ''growth factors'' control animal cell growth and differentiation. Nerve growth factor (or NGF) is involved in the development and survival of neurons, while platelet-derived growth factor (PDGF) participates in blood clotting at the site of a wound. It stimulates the spread of [[fibroblast]]s in the vicinity of the clot, contributing to regrowth of the damaged tissue. | |
== Peptides are an important research tool == | == Peptides are an important research tool == | ||
Revision as of 18:01, 16 March 2007
Peptides are short chains of amino acids linked together via peptide bonds and having a defined sequence. They function primarily as signaling molecules in animals or as antibiotics in some lower organisms.
The number of amino-acid molecules present in a peptide is indicated by a prefix. For example, a dipeptide has two amino acids; a tripeptide, three. An oligopeptide contains a few molecules; a polypeptide, many. Peptides generally contain fewer than 30 amino acid residues, while polypeptides contain as many as 4000. The distinction between polypeptides and proteins is largely academic and imprecise, and the two terms are sometimes used interchangeably. However, there is a movement within the scientific community to define proteins as polypeptides (or complexes of polypeptides) with three-dimensional structure.
The components of peptides
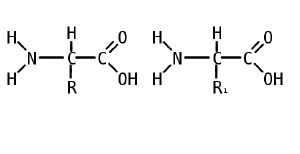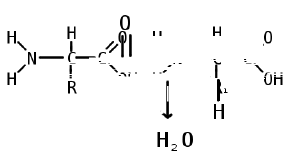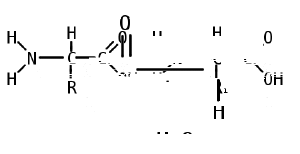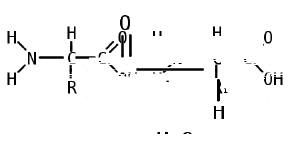
Like proteins, peptides are built from combinations of 20 different amino acids, which are organic molecules composed of an amino group (-NH2), a carboxylic acid group (-COOH), and a unique R group, or side chain. Two amino acids (specifically, alpha-amino acids) are linked together by a peptide bond, which forms when the amino group of one amino acid reacts with the carboxyl group of a second amino acid. An amino acid residue is what is left of an amino acid once it has coupled with another amino acid to form a peptide bond.
Peptides are then created by the polymerization of amino acids, a process in which amino acids are joined together in chains. Shorter strings of amino acids may be referred to as polypeptides, peptides, or, less commonly, oligopeptides.
Peptide synthesis
Peptides are synthesized from amino acids according to an mRNA template, which is itself synthesized from a DNA template inside the cell's nucleus. The precursors of ‘’’ribosomal peptides’’’ are processed in several stages, typically in the endoplasmic reticulum, resulting in "propeptides". These propeptides are then packaged into membrane-bound secretory vesicles, which can be released into the bloodstream in response to specific stimuli.
Nonribosomal peptides, found primarily in unicellular organisms, plants, and fungi, are synthesized using a modular enzyme complex (which functions much like a conveyor belt in a factory). All of these complexes are laid out in a similar fashion, and they can contain many different modules to perform a diverse set of chemical manipulations on the developing peptide. Nonribosomal peptides often have highly complex cyclic structures, although linear nonribosomal peptides are also common.
Some key peptide groups and their biological function
Peptides represent the widest variety of signaling molecules in animals; this group includes peptide hormones, neuropeptides, and polypeptide growth factors. Many peptides are found in both the brain and in nonneural tissues – and can function differently depending on the site. However, blood-brain barrier prevents peptide hormones traveling in the blood from entering the brain, so that peptide hormones do not “confuse” the functioning of the central nervous system.
Peptide hormones
Peptide hormones are a class of peptides that function in living animals as chemical messengers from one cell (or group of cells) to another. Well-known peptide hormones include insulin, glucagons, and the hormones secreted from the pituitary gland, an endocrine gland about the size of a pea that sits in a small, bony cavity at the base of the brain. The latter include follicle stimulating hormone, growth hormone, which acts on bone, muscle and the liver, and vasopressin. Peptide hormones are produced by many different organs and tissues, however, including the heart, pancreas, and gastrointestinal tract.
Neuropeptides
A neuropeptide is any of the variety of peptides found in neural tissue. Approximately 100 different peptides are currently known to be released by different populations of neurons in the mammalian brain. Some neuropeptides may be used as neurotransmitters in the nervous system in addition to acting as hormones when released into the blood.
Neurons use many different chemical signals to communicate information, including neurotransmitters, peptides, cannabinoids, and even some gases, like nitric oxide. Peptide signals play a role in information processing that is different to that of conventional neurotransmitters, and many appear to be particularly associated with specific behaviors. For example, oxytocin and vasopressin have striking and specific effects on social behaviors, including maternal behavior and pair bonding.
Neurotransmitters generally affect the excitability of other neurons, by depolarizing them or by hyperpolarizing them. Peptides have much more diverse effects; among other things, they can affect gene expression, local blood flow, synaptogenesis, and glial cell morphology. Peptides tend to have prolonged actions, and some have striking effects on behavior.
Neurons very often produce both a conventional neurotransmitter (such as glutamate, GABA or dopamine) and one or more neuropeptides. Peptides are generally packaged in large dense-core vesicles, while the co-existing neurotransmitters are contained in small synaptic vesicles.
Vasopressin and oxytoxin
Arginine vasopressin (AVP), also known as argipressin or antidiuretic hormone (ADH), is a human hormone that is mainly released when the body is low on water; it causes the kidneys to conserve water by concentrating the urine and reducing urine volume. Vasopressin released within the brain has many actions:
- It has been implicated in memory formation, including delayed reflexes, image, short- and long-term memory, though the mechanism remains unknown, and these findings are controversial.
- Vasopressin released from centrally-projecting hypothalamic neurons is involved in aggression, blood pressure regulation and temperature regulation.
In recent years, there has been particular interest in the role of vasopressin in social behavior. It is thought that vasopressin, released into the brain during sexual activity, initiates and sustains patterns of activity that support the pair-bond between the sexual partners; in particular, vasopressin seems to induce the male to become aggressive towards other males. Evidence for this comes from experimental studies, in several species, which indicate that the precise distribution of vasopressin and vasopressin receptors in the brain is associated with species-typical patterns of social behavior. In particular, there are consistent differences between monogamous species and promiscuous species in the distribution of vasopressin receptors, and sometimes in the distribution of vasopressin-containing axons, even when closely-related species are compared. Moreover, studies involving either injecting vasopressin agonists into the brain, or blocking the actions of vasopressin, support the hypothesis that vasopressin is involved in aggression towards other males. There is also evidence that differences in the vasopressin receptor gene between individual members of a species might be predictive of differences in social behavior.
Oxytocin (Greek: "quick birth") is a mammalian hormone that also acts as a neurotransmitter in the brain. In women, it is released mainly after distension of the cervix and vagina during labor, and after stimulation of the nipples, facilitating birth and breastfeeding, respectively. Oxytocin, like vasopressin, is released during orgasm in both sexes. In the brain, oxytocin is involved in social recognition and bonding, and might be involved in the formation of trust between people.
Opioid peptides
Opioid peptides mimic the effects of morphine; they work by binding to opioid receptors, which are found principally in the central nervous system and the gastrointestinal tract. Opioid peptides that are produced in the body include endorphins and enkephalins, which inhibit the transmission of pain impulses; they act as natural pain killers, or opiates, and decrease pain responses in the central nervous system.
When certain food proteins such as gluten, casein, egg protein, and spinach protein are broken down, opioid peptides are released. Individuals that are unable to break them down fully may suffer from developmental disorders, such as autism. A gluten-free casein-free diet (or GFCF diet) is a restrictive diet which entirely eliminates intake of the naturally-occuring proteins gluten (found naturally in wheat, barley, and rye), and casein (found in milk). The GFCF diet is recommended by advocacy groups, such as the Autism Research Institute. While multiple success stories have been published and circulated, results from clinical trials and double-blind studies have produced little to confirm the diet's efficacy, and the proposed link is regarded as either incorrect, incomplete, or requiring further investigation.[1]
Growth factors
Polypeptide growth factors control animal cell growth and differentiation. Nerve growth factor (or NGF) is involved in the development and survival of neurons, while platelet-derived growth factor (PDGF) participates in blood clotting at the site of a wound. It stimulates the spread of fibroblasts in the vicinity of the clot, contributing to regrowth of the damaged tissue.
Peptides are an important research tool
Peptides have received prominence in molecular biology in recent times for several reasons:
- Peptides allow researchers to generate antibodies in animals without the need to purify the protein of interest. The researcher can simply make antigenic peptides of sections of the protein.
- Peptides have become instrumental in mass spectrometry, allowing the identification of proteins of interest based on peptide masses and sequences.
- Peptides have recently been used in the study of protein structure and function. Peptide fragments are components of proteins that research use to identify or quantify the source protein. Often these fragments are the products of enzymatic degradation performed in the laboratory on a controlled sample, but they can also be forensic or paleontological samples that have been degraded by natural effects. In addition, synthetic peptides can be used as probes to determine where protein-peptide interactions occur.
- Inhibitory peptides are also used in clinical research to examine the effects of peptides on the inhibition of cancer proteins and other diseases.
Peptide families
Below is a more detailed list of the major families of ribosomal peptides:
Vasopressin and oxytocin
- Vasopressin
- Oxytocin
The Tachykinin peptides
- Substance P
- Kassinin
- Neurokinin A
- Eledoisin
- Neurokinin B
Vasoactive intestinal peptides
- VIP Vasoactive intestinal peptide
- PACAP Pituitary adenylate cyclase activating peptide
- PHI 27
- PHM 27
- GHRH 1-24 Growth hormone releasing hormone 1-24
- Glucagon
- Secretin
Pancreatic polypeptide-related peptides
- NPY
- PYY Peptide YY
- APP Avian pancreatic polypeptide
- HPP Human pancreatic polypeptide
Opioid peptides
- Proopiomelanocortin (POMC) Peptides
- The Enkephalin pentapeptides
- The Prodynorphin peptides
Calcitonin peptides
- Calcitonin
- Amylin
- AGG01
ReferencesISBN links support NWE through referral fees
- Cooper, G.M. & R.E. Hausman. 2004. The Cell: A Molecular Approach, 3rd edition. Washington, D.C.: ASM Press & Sunderland, M.A.: Sinauer Associates.
- Lodish, H., Baltimore, D., Berk, A., Zipursky, S.L., Matsudaira, P., & J. Darnell. 1995. Molecular Cell Biology, 3rd edition. New York, NY: Scientific American Books.
- Stryer, L. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Elder, J., et al. 2006. "The Gluten-Free, Casein-Free Diet in Autism: Results of a Preliminary Double Blind Clinical Trial." Journal of Autism and Developmental Disorders 36:413-420.