Methylene blue
((Started))
| Methylene blue | |
|---|---|
| |
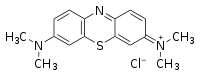
| Chemical name | 3,7-bis(Dimethylamino)- phenazathionium chloride Tetramethylthionine chloride |
| Chemical formula | C16H18N3ClS |
| Molecular mass | 319.85 g/mol |
| CAS number | [61-73-4] |
| EC number | 200-515-2 |
| Density | ? g/cm³ |
| Melting point | 100 °C |
| Boiling point | Decomposes |
| SMILES | CN(C)c3ccc2nc1ccc(N(C) C)cc1[s+]c2c3.[Cl-] |
| Disclaimer and references | |
Methylene blue is a heterocyclic aromatic chemical compound with molecular formula: C16H18ClN3S. It has many uses in a range of different fields, such as biology or chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in water. Methylene blue should not be confused with methyl blue, another histology stain, new methylene blue, nor with the methyl violets often used as pH indicators.
Uses
Chemistry
Methylene blue is widely used a redox indicator in analytical chemistry. Solutions of this substance are blue when in an oxidizing environment, but will turn colorless if exposed to a reducing agent. The redox properties can be seen in a classical demonstration of chemical kinetics in general chemistry, the "blue bottle" experiment. Typically, a solution is made of dextrose, methylene blue, and sodium hydroxide. Upon shaking the bottle, oxygen oxidizes methylene blue, and the solution turns blue. The dextrose will gradually reduce the methylene blue to its colorless, reduced form. Hence, when the dissolved oxygen is entirely consumed, the solution will turn colorless. Methylene blue is also used to make the reaction between Fehling's solution and reducing sugars more visible.
Biology
In biology methylene blue is used as a dye for a number of different staining procedures, such as Gram's stain, Wright's stain, and Jenner's stain. Since it is a temporary staining technique, methylene blue can also be used to examine RNA or DNA under the microscope or in a gel: as an example, a solution of methylene blue can be used to stain RNA on hybridization membranes in northern blotting to verify the amount of nucleic acid present. While methylene blue is not as sensitive as ethidium bromide, it is less toxic and it does not intercalate in nucleic acid chains, thus avoiding interference with nucleic acid retention on hybridization membranes or with the hybridization process itself.
It can also be used as an indicator to determine if a cell such as yeast is alive or not. The blue indicator turns colourless in the presence of active enzymes, thus indicating living cells. However if it stays blue it doesn't mean that the cell is dead - the enzymes could be inactive/denatured. It must be noted that methylene blue can inhibit the respiration of the yeast as it picks up hydrogen ions made during the process. The yeast cell cannot then use those ions to release energy.
Medicine
Owing to its reducing agent properties, methylene blue is employed as a medication for the treatment of methemoglobinemia, which can arise from ingestion of certain pharmaceuticals or broad beans. Basically, methylene blue acts to reduce the heme group from methemoglobin to hemoglobin, however since methylene blue is toxic, any methemoglobinemia treatment with this substance should be strictly evaluated by a doctor.
Methylene blue also blocks accumulation of cyclic guanosine monophosphate (cGMP) by inhibiting the enzyme guanylate cyclase: this action results in reduced responsiveness of vessels to cGMP-dependent vasodilators like nitric oxide and carbon monoxide.
Methylene blue is used in endoscopic polypectomy as an adjunct to saline or epinephrine, and is used for injection into the submucosa around the polyp to be removed. This allows the submucosal tissue plane to be identified after the polyp is removed, which is useful in determining if more tissue needs to be removed, or if there has been a high risk for perforation. Methylene blue is also used as a dye in chromoendoscopy, and is sprayed onto the mucosa of the gastrointestinal tract in order to identify dysplasia, or pre-cancerous lesions.
Methylene blue was used at the end of the century as a successful treatment for malaria. It disappeared as an anti-malarial during the wars in Asia, as U.S. soldiers disliked its two inevitable, fully reversible side effects: green urine and blue sclera. Interest in its use has recently been revived,[1] especially because it is very cheap. Several clinical trials are in progress, trying to find a suitable drug combination. Initial attempts to combine methylene blue with chloroquine were disappointing;[2] however, more recent attempts have appeared more promising.
Since its reduction potential is similar to that of oxygen and can be reduced by components of the electron transport chain, large doses of methylene blue are sometimes used as an antidote to cyanide poisoning.
Methylene blue is probably a monoamine oxidase inhibitor, and if infused intravenously at doses exceeding 5 mg/kg, may precipitate serious serotonin toxicity, serotonin syndrome, if combined with any selective serotonin reuptake inhibitors (SSRIs) or other serotonin reuptake inhibitor (e.g., duloxetine, sibutramine, venlafaxine, clomipramine, imipramine).[3]
Aquaculture
Methylene blue is used in aquaculture and by tropical fish hobbyists as a bacterial and fungal infection preventative on freshwater fish eggs. It is also commonly used as a additive in a solution in which infected (or newly purchased) fish are dipped (commonly called a dip). In the dip, the methylene blue serves to fight and kill the offending organisms, as well as increase the oxygen carrying capacity of the fish's hemoglobin. [4] While some hobbyists use methylene blue to treat fish infected with ich, the parasitic[protozoa]] Ichthyophthirius multifiliis, methylene blue has shown to not be the most effective solution, and other solutions, such as ionized copper, are instead suggested. [5] While methylene blue is non-toxic to fish if used in the proper dosage, it is toxic to live plants, and also will harm fish if placed in long-term contact with them.
Misuse
While methylene blue has many uses in the medicine, it has also been used inappropriately by pranksters, due to it's ability to alter urine with a green-blue color. About 75% of an oral dose of methylene blue is released into the urine (mostly in its reduced form - leucomethylene blue), thereby adding a blue-green hue to the urine (some of the remaining methylene blue is excreted via the bile). However, use of this substance in such a way is dangerous, because methylene blue is a biologically active substance[6], and if administered inappropriately, can lead to a number of health complications, including gastrointestinal disturbances and dysuria. Large doses of methylene blue can can produce methaemoglobinaemia, as well as chest pain, dyspnoea, restlessness, apprehension, tremors, a sense of oppression, urinary tract irritation, as well as a mild haemolysis with moderate hyperbilirubinaemia, reticulosis and slight anaemia.[7]
See also
- Prussian blue
- Gentian violet
Notes
- ↑ Schirmer H, Coulibaly B, Stich A, et al. (2003). Methylene blue as an antimalarial agent—past and future. Redox Rep. 8: 272–76.
- ↑ Meissner PE, Mandi G, Coulibaly B, et al. (2006). Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malaria Journal 5: 84.
- ↑ Gillman PK. (2006). Methylene Blue implicated in potentially fatal serotonin toxicity. Anaesthesia 61: 1013-14.
- ↑ http://www.aquaria.info/index.php?name=PNphpBB2&file=viewtopic&p=344037
- ↑ http://www.wetwebmedia.com/methblueart.htm
- ↑ http://www.madsci.org/posts/archives/may99/925860441.Me.r.html
- ↑ http://www.medsafe.govt.nz/profs/datasheet/m/MethyleneBlueinj.htm
ReferencesISBN links support NWE through referral fees
<<This article needs at least 3 reliable references, properly formatted.>>
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.