Fatty acid
| Types of Fats in Food |
|---|
|
| See Also |
Fatty acids are a class of compounds containing a long hydrocarbon chain and a terminal carboxylate group (-COOH). They have the general structure CH3(CH2)nCOOH. Fatty acids belong to a diverse class of biological molecules called lipids, which are generally water-insoluble but highly soluble in organic solvents.
Fatty acids function as fuel molecules and serve as components of many other classes of lipids, such as phospholipids and glycolipids, important building blocks of biological membranes. Fatty acid derivatives also serve as hormones and intracellular messengers.
Fatty acids can be either saturated or unsaturated:
- Saturated fatty acids have no double bonds between the carbon atoms of the fatty acid chain (hence, they are fully saturated with hydrogen atoms).
- Unsaturated fatty acids have one or more double bonds. The presence of double bonds generally reduces the melting point of fatty acids, enhancing the fluidity of unsaturated fatty acids and their derivatives.
Make some kind of diet/bio-function point about degree of unsaturation and fatty acids generally
Chemical structure of fatty acids
Fatty acids are distinguished by two important characteristics: (1) chain length and (2) degree of unsaturation. These qualities affect chem. Properties/function
Chain length
Fatty acid chains in naturally occurring triglycerides are typically unbranched and range from 14 to 24 carbon atoms, with 16- and 18-carbon lengths being the most common. Fatty acids found in plants and animals are usually composed of an even number of carbon atoms, because their biosynthesis in these organisms involves acetyl-CoA, a coenzyme carrying a two-carbon-atom group. Bacteria, however, possess the ability to synthesize odd- and branched-chain fatty acids. Consequently, ruminant animal fat, such as in cattle, contains significant proportions of branched-chain fatty acids, due to the action of bacteria in the rumen.
Fatty acids with long chains are more susceptible to intermolecular forces of attraction (in this case, van der Waals forces), raising their melting point. Long chains also yield more energy per molecule when metabolized.
Degree of unsaturation
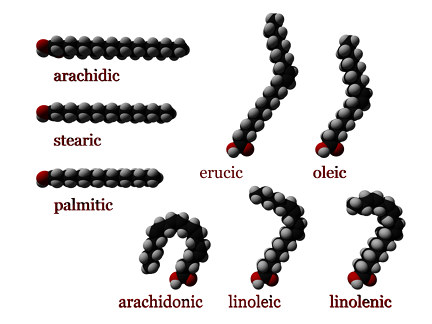
Fatty acids may also differ in the number of hydrogen atoms that branch off of the chain of carbon atoms.
Saturated fatty acids
When each carbon atom in the chain is bonded to two hydrogen atoms, the fatty acid is said to be saturated. Saturated fatty acids do not contain any double bonds between carbon atoms, because the carbon molecules are "saturated” with hydrogen; that is, they are bonded to the maximum number of hydrogen atoms. Saturated fatty acids form straight chains and, as a result, can be packed together very tightly, allowing living organisms to store chemical energy very densely.
Unsaturated fatty acids
Monounsaturated fatty acids contain one double bond near the middle of the chain, creating a "kink" in the chain. One of the carbon atoms, bonded to only one hydrogen atom, forms a double bond with a neighboring carbon atom.
Polyunsaturated fatty acids may contain between two and six double bonds, resulting in multiple "kinks." As the degree of unsaturation increases, the melting points of polyunsaturated fatty acids become lower.
The double bonds in unsaturated fatty acids may occur either in a cis or trans isomer, depending on the geometry of the double bond. In the cis conformation, the hydrogens are on the same side of the double bond, whereas in the trans conformation, they are on the opposite side.
cis : A cis configuration means that the two carbons are on the same side of the double bond. The rigidity of the double bond freezes its conformation and, in the case of the cis isomer, causes the chain to bend and restricts the conformational freedom of the fatty acid. The more double bonds the chain has in the cis configuration, the less flexibility it has. When a chain has many cis bonds, it becomes quite curved in its most accessible conformations. For example, oleic acid, with one double bond, has a "kink" in it, while linoleic acid, with two double bonds, has a more pronounced bend. Alpha-linolenic acid, with three double bonds, favors a hooked shape. The effect of this is that in restricted environments, such as when fatty acids are part of a phospholipid in a lipid bilayer, or triglycerides in lipid droplets, cis bonds limit the ability of fatty acids to be closely packed and therefore could affect the melting temperature of the membrane or of the fat.
A trans configuration, by contrast, means that the next two carbon atoms are bound to opposite sides of the double bond. As a result, they don't cause the chain to bend much, and their shape is similar to straight saturated fatty acids.
A trans fatty acid (commonly shortened to trans fat) is an unsaturated fatty acid molecule that contains a trans double bond between carbon atoms, which makes the molecule less 'kinked' in comparison to fatty acids with cis double bonds. These bonds are characteristically produced during industrial hydrogenation of plant oils. Research suggests that amounts of trans fats correlate with circulatory diseases such as atherosclerosis and coronary heart disease more than the same amount of non-trans fats, for reasons that are not well understood.
In most naturally occurring unsaturated fatty acids, each double bond has 3n carbon atoms after it, for some n, and all are cis bonds. Most fatty acids in the trans configuration (trans fats) are not found in nature and are the result of human processing (eg, hydrogenation).
The differences in geometry between the various types of unsaturated fatty acids, as well as between saturated and unsaturated fatty acids, play an important role is biological processes, and in the construction of biological structures (such as cell membranes).
Fatty acids are important components of membrane lipids
The cell membrane (or plasma membrane) is the thin outer layer of the cell that differentiates the cell from its environment.
As a semi-permeable barrier, the cell membrane maintains an essential balance between individual distinctness and communal interaction: it functions to retain key components of the cell and to keep out toxic or unwanted substances, while selectively controlling the flow of nutrients and biochemical signals into the cell.
The cell membrane is composed mainly of lipid and protein molecules arranged in organized but flexible sheets. The lipid components form a bilayer that contributes structural stability and creates the semi-permeable environment, while the proteins are responsible for most of the dynamic processes carried out by cell membranes, such as the transport of molecules into and out of the cell.
Phospholipids are the major constituents of biological membranes, such as the cell's plasma membrane and the intracellular membranes of organelles. They are derived either from glycerol, a three-carbon alcohol, or sphingosine, a more complex alcohol. The former, called phosphoglycerides (or glycerophospholipids) consist of a glycerol backbone, two fatty acid chains, and a phosphorylated alcohol.
Fatty acids function as a fuel source
When they are not attached to other molecules, fatty acids are known as "free" fatty acids. They may derive from the breakdown of a triglyceride into its fatty acids and glycerol components. Free fatty acids are an important source of fuel for many tissues since they can yield relatively large quantities of ATP. Although many cell types can use either glucose or fatty acids for fuel, heart and skeletal muscle prefer fatty acids. On the other hand, the brain cannot use fatty acids as a source of fuel. During starvation or periods of low carbohydrate intake, the brain relies instead on glucose or on ketone bodies produced by the liver from fatty acid metabolism.
Triglyceride is the storage form of fatty acids
Triglycerides play an important role in metabolism as highly concentrated energy stores; when metabolized, they yield more than twice as much energy as carbohydrates and proteins (approximately 9 kcal/g versus 4 kcal/g). Triglycerides make such efficient energy stores because they are (1) highly reduced and (2) nearly anhydrous (because they are relatively nonpolar, they do not need to be stored in hydrated form).
In animals, a type of loose connective tissue called adipose contains adipocytes, specialized cells that form and store droplets of fat. Depending on the animal's current physiological conditions, adipocytes either store fat derived from the diet and liver or degrade stored fat to supply fatty acids and glycerol to the circulation. When energy is needed, stored triglycerides are broken down to release glucose and free fatty acids.
Fatty acid derivatives serve as hormones and intracellular messengers
The human body can produce all but two of the fatty acids it needs. These two, linoleic acid and alpha-linolenic acid, are widely distributed in plant and fish oils. Since they cannot be made in the body from other substrates and must be supplied in food, they are called essential fatty acids. In the body, essential fatty acids are primarily used to produce hormone-like substances that regulate a wide range of functions, including blood pressure, blood clotting, blood lipid levels, the immune response, and the inflammation response to injury infection.
Essential fatty acids are polyunsaturated fatty acids and are the parent compounds of the omega-6 and omega-3 fatty acid series, respectively. They are essential in the human diet because there is no synthetic mechanism for them. Humans can easily make saturated fatty acids or monounsaturated fatty acids with a double bond at the omega-9 position, but do not have the enzymes necessary to introduce a double bond at the omega-3 or omega-6 position.
The essential fatty acids are important in several human body systems, including the immune system and in blood pressure regulation, since they are used to make compounds such as prostaglandins. The brain has increased amounts of linolenic and alpha-linoleic acid derivatives. Changes in the levels and balance of these fatty acids due to a typical Western diet rich in omega-6 and poor in omega-3 fatty acids is alleged to be associated with depression and behavioral change, including violence. The actual connection, if any, is still under investigation. Further, changing to a more natural diet, or consumption of supplements to compensate for a dietary imbalance, has been associated with reduced violent behavior[1] and increased attention span, but the mechanisms for the effect are still unclear. So far, at least three human studies have shown results that support this: two school studiesTemplate:Citeneeded[2] as well as a double blind study in a prison.[1][3][4]
Related topics
Fatty acids in the diet
Naturally occurring fats contain varying proportions of saturated and unsaturated fatty acids, which in turn determine their relative energy content and melting point. The following table gives the fatty acid and cholesterol composition of some common dietary fats.[5] [6]
| Saturated | Monounsaturated | Polyunsaturated | Cholesterol | Vitamin E | |
|---|---|---|---|---|---|
| g/100g | g/100g | g/100g | mg/100g | mg/100g | |
| Animal fats | |||||
| Lard | 40.8 | 43.8 | 9.6 | 93 | 0.00 |
| Butter | 54.0 | 19.8 | 2.6 | 230 | 2.00 |
| Vegetable fats | |||||
| Coconut oil | 85.2 | 6.6 | 1.7 | 0 | .66 |
| Palm oil | 45.3 | 41.6 | 8.3 | 0 | 33.12 |
| Cottonseed oil | 25.5 | 21.3 | 48.1 | 0 | 42.77 |
| Wheat germ oil | 18.8 | 15.9 | 60.7 | 0 | 136.65 |
| Soya oil | 14.5 | 23.2 | 56.5 | 0 | 16.29 |
| Olive oil | 14.0 | 69.7 | 11.2 | 0 | 5.10 |
| Corn oil | 12.7 | 24.7 | 57.8 | 0 | 17.24 |
| Sunflower oil | 11.9 | 20.2 | 63.0 | 0 | 49.0 |
| Safflower oil | 10.2 | 12.6 | 72.1 | 0 | 40.68 |
| Rapeseed oil | 5.3 | 64.3 | 24.8 | 0 | 22.21 |
Nomenclature
In IUPAC nomenclature, the name of a fatty acid is derived from its parent hydrocarbon by substituting the suffix -oic for the final -e. (In common nomenclature, the suffix is usually -ic.) For example, —
The notation C18:0 means that the carbon chain of the fatty acid consists of 18 carbon atoms and does not contain any double bonds, whereas the notation C18:1 describes an 18-carbon chain with one double bond.
There are two methods for describing the position of a double bond in the hydrocarbon chain:
- cis/trans-Delta-x or cis/trans-Δx: The double bond is located on the xth carbon-carbon bond, counting from the carboxyl end. The cis or trans notation indicates whether the molecule is arranged in a cis or trans conformation. In the case of a molecule having more than one double bond, the notation is, for example, cis,cis-Δ9,Δ12.
- Omega-x or ω-x : Alternatively, the position of a double bond can be counted by starting from the distal end, with the ω carbon (methyl carbon) as position one. Sometimes, the symbol ω is substituted with a lowercase letter n, making the notation n-6 or n-3.
ReferencesISBN links support NWE through referral fees
- Krogh, D. 2005. Biology: A Guide to the Natural World, 3rd edition. Upper Saddle River, NJ: Pearson.
- Purves, W., D. Sadava, G. Orians, & H. C. Heller. 2004. Life: The Science of Biology, 7th edition. Sunderland, MA: Sinauer.
- Stryer, L. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
- Chemical Structure of Fats and Fatty Acids
- Plant Oils and Fats, from the Cyberlipid Center Web site
- Fat content and fatty acid composition of seed oils. Retrieved 2006-10-07. From Udo Erasmus' book, Fats that Heal Fats that Kill
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ 1.0 1.1 C. Bernard Gesch, CQSW Sean M. Hammond, PhD Sarah E. Hampson, PhD Anita Eves, PhD Martin J. Crowder, PhD (2002). Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. The British Journal of Psychiatry 181: 22-28.
- ↑ Alexandra J. Richardson and Paul Montgomery (2005). The Oxford-Durham study: a randomized controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 115 (5): 1360 - 1366.
- ↑ Lawrence, Felicity (2004). in Kate Barker: Not on the Label. Penguin, 213. ISBN 0-14-101566-7.
- ↑ Using Fatty Acids for Enhancing Classroom Achievement. Retrieved January, 2004.
- ↑ Food Standards Agency (1991). "Fats and Oils", McCance & Widdowson's The Composition of Foods. Royal Society of Chemistry.
- ↑ Ted Altar. More Than You Wanted To Know About Fats/Oils. Sundance Natural Foods Online. Retrieved 2006-08-31.