Botulinum toxin
| |
Botulinum toxin
| |
| Systematic name | |
| IUPAC name ? | |
| Identifiers | |
| CAS number | 93384-43-1 |
| ATC code | M03AX01 |
| PubChem | ? |
| DrugBank | BTD00092 |
| Chemical data | |
| Formula | C6760H10447N1743O2010S32 |
| Mol. weight | 149320.83328 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | ? |
| Routes | IM (approved),SC, intradermal, into glands |
Botulinum toxin is any of several distinct types of a neurotoxin protein produced by the bacterium Clostridium botulinum. Botulinum toxin is one of the most poisonous naturally occurring substances in the world. It the most toxic protein known (Montecucco and Molgó 2005), and perhaps the most acutely toxic substance.
Though it is highly toxic to humans and can cause botulism, botulinum toxin also is used medicinally in minute doses to block excessive and inappropriate muscle contractions and other therapeutic applications as well as cosmetically, such as to smooth facial lines and wrinkles (McClain 2002).
Botulinum toxin is sold commercially under the brand names Botox, Dysport, and Myobloc for cosmetic purpose. The terms Botox, Dysport, and Myobloc are trade names and are not used generically to describe the neurotoxins produced by C. botulinum.
Source: Clostridium botulinum
Clostridium botulinum is a Gram-positive, rod-shaped bacterium that produces the neurotoxin botulin (botulinum toxin). It is an obligate anaerobe, meaning that oxygen is poisonous to the cells. However, they tolerate very small traces of oxygen due to an enzyme called superoxide dismutase (SOD) which is an important antioxidant defense in nearly all cells exposed to oxygen. Under unfavorable circumstances, they are able to form endospores that allow them to survive in a dormant state until exposed to conditions that can support their growth (Beuchat and Doyle 2007).
Chemical overview and lethality
Botulinum toxin, or botulin, is a two-chain polypeptide with a 100-kDa heavy chain joined by a disulfide bond to a 50-kDa light chain. This light chain is an enzyme (a protease) that attacks one of the fusion proteins (SNAP-25, syntaxin or synaptobrevin) at a neuromuscular junction, preventing vesicles from anchoring to the membrane to release acetylcholine. By inhibiting acetylcholine release, the toxin interferes with nerve impulses and causes flaccid (sagging) paralysis of muscles as seen in botulism, as opposite to the spastic paralysis seen in tetanus.
There are seven serologically distinct toxin types, designated A through G. Three subtypes of A have been described.
Botulinum toxin is possibly the most acutely toxic substance known, with a median lethal dose of about 1 nanogram per kilogram (ng/kg) (Arnon et al. 2001), meaning that a few hundred grams could theoretically kill every human on earth. (For perspective, the rat poison strychnine, often described as highly toxic, has an LD50 of 1,000,000 ng/kg, and would thus take about six metric tons to kill every human.)
Botulinum toxin is also remarkably easy to come by: Clostridium spores are found in soil practically all over the earth.
Food-borne botulism usually results from ingestion of food that has become contaminated with spores (such as a perforated can) in an anaerobic environment, allowing the spores to germinate and grow. The growing (vegetative) bacteria produce toxin. It is the ingestion of preformed toxin that causes botulism, not ingestion of the spores or vegetative organism.
Infant (intestinal) and wound botulism both result from infection with spores that subsequently germinate, resulting in production of toxin and the symptoms of botulism.
The toxin itself is rapidly destroyed by heat, such as in thorough cooking (Licciardello et al. 1967). However, the spores that produce the toxin are heat-tolerant and will survive boiling at 100 degrees Celsius for an extended period of time (Setlowa 2007).
Biochemical mechanism of toxicity
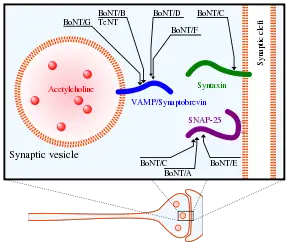
The heavy chain of the toxin is particularly important for targeting the toxin to specific types of axon terminals. The toxin must get inside the axon terminals in order to cause paralysis. Following the attachment of the toxin heavy chain to proteins on the surface of axon terminals, the toxin can be taken into neurons by endocytosis. The light chain is able to leave endocytotic vesicles and reach the cytoplasm. The light chain of the toxin has protease activity. The type A toxin proteolytically degrades the SNAP-25 protein, a type of SNARE protein. The SNAP-25 protein is required for the release of neurotransmitters from the axon endings.[1] Botulinum toxin specifically cleaves these SNAREs, and so prevents neuro-secretory vesicles from docking/fusing with the nerve synapse plasma membrane and releasing their neurotransmitters.
Though it affects the nervous system, common nerve agent treatments (namely the injection of atropine and 2-pam-chloride) will increase mortality by enhancing botulin toxin's mechanism of toxicity. Attacks involving botulinum toxin are distinguishable from those involving nerve agent in that NBC detection equipment (such as M-8 paper or the ICAM) will not indicate a "positive" when a sample of the agent is tested. Furthermore, botulism symptoms develop relatively slowly, over several days compared to nerve agent effects, which can be instantaneous.
Medical uses
Researchers discovered in the 1950s that injecting overactive muscles with minute quantities of botulinum toxin type A decreased muscle activity by blocking the release of acetylcholine at the neuromuscular junction, thereby rendering the muscle unable to contract for a period 3 to 4 months.[citation needed]
Alan Scott, a San Francisco ophthalmologist, first applied tiny doses of the toxin in a medicinal sense to treat 'crossed eyes' (strabismus) and 'uncontrollable blinking' (blepharospasm), but needed a partner to gain regulatory approval to market his discovery as a drug. Allergan, Inc., a pharmaceutical company that focused on prescription eye therapies and contact lens products, bought the rights to the drug in 1988 and received FDA approval in 1989.[citation needed] Allergan renamed the drug Botox.
Cosmetically desirable effects of Botox were first discovered by Vancouver-based cosmetic surgeons Drs. Alastair and Jean Carruthers[citation needed] The serendipitous discovery occurred when the husband-and-wife team observed the softening of patients' frown lines following treatment for eye muscle disorders, leading to clinical trials and subsequent FDA approval for cosmetic use in April 2002.[citation needed] As of 2007, Botox injection is the most common cosmetic operation, with 4.6 million procedures in the United States, according to the American Society of Plastic Surgeons.
Besides its cosmetic application, Botox is used in the treatment of
- migraine headaches
- cervical dystonia (spasmodic torticollis) (a neuromuscular disorder involving the head and neck)[2]
- blepharospasm (involuntary blinking)[3]
- severe primary axillary hyperhidrosis (excessive sweating)[4]
- Achalasia (failure of the lower esophageal sphincter to relax)
Other uses of botulinum toxin type A that are widely known but not specifically approved by FDA include treatment of:
- pediatric incontinence[5], incontinence due to overactive bladder,[6] and incontinence due to neurogenic bladder.[7]
- anal fissure[8]
- spastic disorders associated with injury or disease of the central nervous system including trauma, stroke, multiple sclerosis, Parkinson's disease, or cerebral palsy
- focal dystonias affecting the limbs, face, jaw, or vocal cords
- TMJ pain disorders
- diabetic neuropathy
- wound healing
- excessive salivation
- VCD Vocal cord dysfunction a spasming of the vocal cords
- Reduction of the Masseter muscle for decreasing the size of the lower jaw
Treatment and prevention of chronic headache[9] and chronic musculoskeletal pain[10] are emerging uses for botulinum toxin type A. In addition, there is evidence that Botox may aid in weight loss by increasing the gastric emptying time.[11]
Links to deaths
On September 2005, a paper published in the Journal of American Academy of Dermatology reported from the FDA saying that use of Botox has resulted in 28 deaths between 1989 and 2003, though none were attributed to cosmetic use.[12]
On February 8, 2008, the FDA announced that Botox has "been linked in some cases to adverse reactions, including respiratory failure and death, following treatment of a variety of conditions using a wide range of doses," due to its ability to spread to areas distant to the site of the injection.[13]
Several cases of death have been linked to the use of fake Botox.[14]
Side effects
Side effects can be predicted from the mode of action (muscle paralysis) and chemical structure (protein) of the molecule, resulting broadly speaking in two major areas of side effects: paralysis of the wrong muscle group and allergic reaction. Bruising at the site of injection is a side effect not of the toxin, but rather the mode of administration. In cosmetic use, this means that the client will complain of inappropriate facial expression such as drooping eyelid, uneven smile, loss of ability to close the eye. This will wear off in around 6 weeks. Bruising is prevented by the clinician applying pressure to the injection site, but may still occur, and will last around 7 - 10 days. When injecting the masseter muscle of the jaw, loss of muscle function will result in a loss or reduction of power to chew solid foods. All cosmetic treatments are of limited duration, and can be as short a period as six weeks, but usually one reckons with an effective period of between 3 and 8 months. At the extremely low doses used medicinally, botulinum toxin has a very low degree of toxicity.
Reported adverse events from cosmetic use includes headaches, focal facial paralysis, muscle weakness, dysphagia, flu-like syndromes, and allergic reactions[12].
There has been a petition by Public Citizen to the FDA requesting regulatory action concerning the possible spread of botulinum toxin (Botox, Myobloc) from the site of injection to other parts of the body (HRG Publication #1834): Public Citizen
Treatment of botulinum poisoning
The case fatality rate for botulinum poisoning between 1950 and 1996 was 15.5%, down from approximately 60% over the previous 50 years.[15] Death is generally secondary to respiratory failure due to paralysis of the respiratory muscles, so treatment consists of antitoxin administration and artificial ventilation. If initiated on time, these are quite effective. Occasionally, functional recovery may take several weeks to months.
There are two primary Botulinum Antitoxins available for treatment of botulism.
- Trivalent (A,B,E) Botulinum Antitoxin is derived from equine sources utilizing whole antibodies (Fab & Fc portions). This antitoxin is available from the local health department via the CDC.
- The second antitoxin is Heptavalent (A,B,C,D,E,F,G) Botulinum Antitoxin which is derived from "despeciated" equine IgG antibodies which have had the Fc portion cleaved off leaving the F(ab')2 portions. This is a less immunogenic antitoxin that is effective against all known strains of botulism where not contraindicated. This is available from the US Army. On June 1, 2006 the US Department of Health and Human Services awarded a $363 million contract with Cangene Corporation for 200,000 doses of Heptavalent Botulinum Antitoxin over five years for delivery into the Strategic National Stockpile beginning in 2007.[16]
History
Between 1817 and 1822 the German physician and poet Justinus Kerner described botulinium toxin, using the terms "sausage poison" and "fatty poison"[17], as this bacterium often causes poisoning by growing in badly handled or prepared meat products. He first conceived a possible therapeutic use of botulinium toxin. In 1870, Müller (another German physician) coined the name botulism, from Latin botulus = "sausage". In 1895, Emile van Ermengem first isolated the bacterium Clostridium botulinum. In 1944, Edward Schantz cultured Clostridium botulinum and isolated the toxin, and, in 1949, Burgen's group discovered that botulinum toxin blocks neuromuscular transmission.
By 1973, Alan B Scott, MD, of Smith-Kettlewell Institute used botulinium toxin type A (BTX-A) in monkey experiments, and, in 1980, he officially used BTX-A for the first time in humans to treat strabismus. In December 1989, BTX-A (BOTOX) was approved by the US Food and Drug Administration (FDA) for the treatment of strabismus, blepharospasm, and hemifacial spasm in patients over 12 years old. The cosmetic effect of BTX-A was initially described by ophthalmologist Jean Carruthers and dermatologist Alastair Carruthers, a husband-and-wife team working in Vancouver, Canada, although the effect had been observed by a number of independent groups. On April 15, 2002, the FDA announced the approval of botulinum toxin type A (BOTOX Cosmetic) to temporarily improve the appearance of moderate-to-severe frown lines between the eyebrows (glabellar lines). BTX-A has also been approved for the treatment of excessive underarm sweating. The acceptance of BTX-A use for the treatment of spasticity and muscle pain disorders is growing, with approvals pending in many European countries and studies on headaches (including migraine), prostatic symptoms, asthma, obesity and many other possible indications are ongoing.
Botox is manufactured by Allergan Inc (U.S.) for both therapeutic as well as cosmetic use. The formulation is best stored at cold temperature of 2-8 degrees Celsius. Dysport is a therapeutic formulation of the type A toxin developed and manufactured in Ireland and which is licenced for the treatment of focal dystonias and certain cosmetic uses in many territories world wide. Neuronox is a new type A toxin manufactured by Medy-Tox Inc (South Korea).
Botulinium Toxin Type B (BTX-B) received FDA approval for treatment of cervical dystonia on December 21, 2000. Trade names for BTX-B are Myobloc in the United States, and Neurobloc in the European Union.
ReferencesISBN links support NWE through referral fees
- ↑ Foran PG, Mohammed N, Lisk GO, et al (2003). Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 278 (2): 1363–71.
- ↑ Brin MF, Lew MF, Adler CH, Comella CL, Factor SA, Jankovic J, O'Brien C, Murray JJ, Wallace JD, Willmer-Hulme A, Koller M (1999). Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-resistant cervical dystonia. Neurology 53 (7): 1431–8.
- ↑ Shukla HD, Sharma SK (2005). Clostridium botulinum: a bug with beauty and weapon. Crit. Rev. Microbiol. 31 (1): 11–8.
- ↑ Eisenach JH, Atkinson JL, Fealey RD (2005). Hyperhidrosis: evolving therapies for a well-established phenomenon. Mayo Clin. Proc. 80 (5): 657–66.
- ↑ Schurch B, Corcos J (2005). Botulinum toxin injections for paediatric incontinence. Current opinion in urology 15 (4): 264–7.
- ↑ Duthie J, Wilson D, Herbison G, Wilson D (2007). Botulinum toxin injections for adults with overactive bladder syndrome. 3: CD005493.
- ↑ Akbar M, Abel R, Seyler TM, Gerner HJ, Möhring K (2007). Repeated botulinum-A toxin injections in the treatment of myelodysplastic children and patients with spinal cord injuries with neurogenic bladder dysfunction.. BJU Int. 100 (3): 639–45.
- ↑ Trzciński R, Dziki A, Tchórzewski M (2002). Injections of botulinum A toxin for the treatment of anal fissures. The European journal of surgery = Acta chirurgica 168 (12): 720–3.
- ↑ Panicker JN, Muthane UB (2003). Botulinum toxins: pharmacology and its current therapeutic evidence for use. Neurology India 51 (4): 455–60.
- ↑ Charles PD (2004). Botulinum neurotoxin serotype A: a clinical update on non-cosmetic uses. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 61 (22 Suppl 6): S11–23.
- ↑ Coskun H, Duran Y, Dilege E, Mihmanli M, Seymen H, Demirkol MO (2005). Effect on gastric emptying and weight reduction of botulinum toxin-A injection into the gastric antral layer: an experimental study in the obese rat model. Obesity surgery : the official journal of the American Society for Bariatric Surgery and of the Obesity Surgery Society of Australia and New Zealand 15 (8): 1137–43.
- ↑ 12.0 12.1 Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM (September 2005). Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J. Am. Acad. Dermatol. 53 (3): 407–15.
- ↑ FDA Notifies Public of Adverse Reactions Linked to Botox Use
- ↑ Woman Dies From Fake Botox Injections
- ↑ Disease Listing, Botulism Manual, Additional Information. Retrieved 2007-08-14.
- ↑ FEMA. Retrieved 2007-08-14.
- ↑ Frank J. Erbguth (2004). Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Movement Disorders 19 (S8): S2–S6.
JAMA. 2001 Feb 28;285(8):1059-70.Click here to read Links
Botulinum toxin as a biological weapon: medical and public health management. Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K; Working Group on Civilian Biodefense.
.[3]
- McClain, B. 2002. Botulinum toxin injections. Pages 558 to 559 in in J.L. Longe (ed.), The Gale Encyclopedia of Medicine, 2nd edition, volume 1. Detroit: Gale Group/Thomson Learning. ISBN 0787654906.
.[4]
External links
- A Poison that can Heal from the FDA
- How Stuff Works - Botox
- Pros and Cons of using Botox for Vagininsmus
- Does Botox get into the brain? Troubling research contradicts earlier findings about the treatment
- Patient Reported Benefit and Satisfaction with Botulinum Toxin Type A Treatment of moderate to severe Glabellar
- BotDB: extensive resources on BoNT structures, inhibitors, kinetics, and literature
Template:Muscle relaxants
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Arnon, Stephen. "Botulinum Toxin as a Biological Weapon." JAMA. vol 285. pp.1059-1070. 2001.
- ↑ Beuchat, Larry R., and Michael P. Doyle. 2007. Food microbiology: fundamentals and frontiers. Washington, D.C.: ASM Press. ISBN 9781555814076
- ↑ Licciardello JJ, Nickerson JT, Ribich CA, Goldblith SA (March 1967). Thermal inactivation of type E botulinum toxin. Appl Microbiol 15 (2): 249–56.
- ↑ Montecucco C, Molgó J (2005). Botulinal neurotoxins: revival of an old killer. Current opinion in pharmacology 5 (3): 274–9.
- ↑ Setlowa, Peter (April 2007). I will survive: DNA protection in bacterial spores. Trends in Microbiology 15 (4): 172–180.