|
|
| (172 intermediate revisions by 2 users not shown) |
| Line 1: |
Line 1: |
| | + | {{Images OK}}{{Submitted}}{{Approved}}{{Copyedited}} |
| | + | [[File:Brain metabolism and drug addiction.jpg|thumb|400px|[[Brain positron emission tomography]] images that compare [[Human brain#Metabolism|brain metabolism]] in a healthy individual and an individual with a [[cocaine]] addiction]] |
| | | | |
| − | '''Currently working on''' —[[User:Jennifer Tanabe|Jennifer Tanabe]] ([[User talk:Jennifer Tanabe|talk]]) May 2020 | + | '''Addiction''' is a [[brain disorder]] characterized by compulsive engagement in [[reward system|rewarding]] stimuli despite adverse consequences. A number of psychosocial factors are involved, but it is a biological process—one that is induced by repeated exposure to an addictive stimulus—that is the core [[pathology]] that drives the development and maintenance of an addiction. Addictive stimuli are [[positive reinforcement|reinforcing]] and [[intrinsic reward|intrinsically rewarding]]. |
| | + | {{toc}} |
| | + | Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences. Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs). Addiction has a massive overall economic cost to society, and, more importantly, is destructive to individuals, their families, and the social well-being of society as a whole. |
| | | | |
| | + | ==Definition== |
| | + | The American Society of Addiction Medicine defines addiction as follows: |
| | + | <blockquote>Addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences. People with addiction use substances or engage in behaviors that become compulsive and often continue despite harmful consequences.<ref name=ASAM>[https://www.asam.org/quality-care/definition-of-addiction What is the definition of addiction?] ''American Society of Addiction Medicine''. Retrieved August 31, 2022.</ref></blockquote> |
| | + | {{readout||right|250px|Addictions can be either to [[substance abuse]] or behaviors that lead to a reward, such as [[gambling]], eating, or sexual activity}} |
| | + | Addiction is a [[brain disorder]] characterized by compulsive engagement in [[reward system|rewarding]] stimuli despite adverse consequences.<ref name=Nestleretal>Eric Nestler, Steven Hyman, and Robert Malenka, ''Molecular Neuropharmacology: A Foundation for Clinical Neuroscience'' (McGraw-Hill, 2008, ISBN 978-0071481274).</ref> The two properties that characterize all addictive stimuli are that they are [[positive reinforcement|reinforcing]] (in other words, they increase the likelihood that a person will seek repeated exposure to them) and [[intrinsic reward|intrinsically rewarding]] (meaning they are perceived as being inherently positive, desirable, and pleasurable).<ref name=Taylor>Sara B. Taylor, Candace R. Lewis, and M. Foster Olive, [https://pubmed.ncbi.nlm.nih.gov/24648786/ The Neurocircuitry of Illicit Psychostimulant Addiction: Acute and Chronic Effects in Humans] ''Subst Abuse Rehabil'' 8(4) (2013):29-43. Retrieved August 31, 2022.</ref> |
| | | | |
| − | [[File:Brain metabolism and drug addiction.jpg|thumb|300px|[[Brain positron emission tomography]] images that compare [[Human brain#Metabolism|brain metabolism]] in a healthy individual and an individual with a [[cocaine]] addiction]]
| + | Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences. Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs).<ref>G.A. Marlatt, J.S. Baer, D.M. Donovan, and D.R. Kivlahan, [https://pubmed.ncbi.nlm.nih.gov/3278676/ Addictive Behaviors: Etiology and Treatment] ''Annual Review of Psychology'' 39 (1988): 223–252. Retrieved August 31, 2022.</ref> |
| − | | |
| − | | |
| − | | |
| − | '''Addiction''' is a [[brain disorder]] characterized by compulsive engagement in [[reward system|rewarding]] stimuli despite adverse consequences.{{refn|<ref name="Cellular basis" /><ref name="Nestler Labs Glossary" /><ref name="Brain disease" /><ref name="pmid18790142">{{cite journal |vauthors=Angres DH, Bettinardi-Angres K | title = The disease of addiction: origins, treatment, and recovery | journal = Disease-a-Month | volume = 54 | issue = 10 | pages = 696–721 |date=October 2008| pmid = 18790142 | doi = 10.1016/j.disamonth.2008.07.002 | url = }}</ref><ref name="NHM addiction-reward-reinforcement" /><ref name="Reward system and psychostimulants" />}} Despite the involvement of a number of psychosocial factors, a biological process—one that is induced by repeated exposure to an addictive stimulus—is the core [[pathology]] that drives the development and maintenance of an addiction.<ref name="Cellular basis" /><ref>{{cite web | author = American Society for Addiction Medicine | title = Definition of Addiction | journal = | volume = | issue = | pages = | year = 2012 | url = http://www.asam.org/for-the-public/definition-of-addiction}}</ref> The two properties that characterize all addictive stimuli are that they are [[positive reinforcement|reinforcing]] (i.e., they increase the likelihood that a person will seek repeated exposure to them) and [[intrinsic reward|intrinsically rewarding]] (i.e., they are perceived as being inherently positive, desirable, and pleasurable).<ref name="Cellular basis" /><ref name="Nestler Labs Glossary" /><ref name="Reward system and psychostimulants" />
| |
| − | | |
| − | Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences.<ref name="pmid1501306">{{cite journal |vauthors=Morse RM, Flavin DK | title = The definition of alcoholism. The Joint Committee of the National Council on Alcoholism and Drug Dependence and the American Society of Addiction Medicine to Study the Definition and Criteria for the Diagnosis of Alcoholism | journal = JAMA | volume = 268 | issue = 8 | pages = 1012–14 |date=August 1992| pmid = 1501306 | doi = 10.1001/jama.1992.03490080086030 }}</ref> Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs).<ref name="pmid3278676">{{cite journal |vauthors=Marlatt GA, Baer JS, Donovan DM, Kivlahan DR | title = Addictive behaviors: etiology and treatment | journal = Annu Rev Psychol | volume = 39 | issue = | pages = 223–52 | year = 1988 | pmid = 3278676 | doi = 10.1146/annurev.ps.39.020188.001255 }}</ref> | |
| − | | |
| − | Addiction exacts an "astoundingly high financial and human toll" on individuals and society as a whole.<ref name="Societal toll">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | page = 4 | edition = 2nd | chapter = Chapter 1: Basic Principles of Neuropharmacology | quote = Drug abuse and addiction exact an astoundingly high financial and human toll on society through direct adverse effects, such as lung cancer and hepatic cirrhosis, and indirect adverse effects –for example, accidents and AIDS – on health and productivity.}}</ref><ref name="Epidem">{{cite journal|vauthors = Merikangas KR, McClair VL | title = Epidemiology of Substance Use Disorders | date = June 2012 | journal = Hum. Genet. | pages = 779–89 | issue = 6 | volume = 131 | pmc = 4408274 | doi = 10.1007/s00439-012-1168-0 | pmid = 22543841 }}</ref><ref name="ABAM" /> In the United States, the total economic cost to society is greater than that of all types of [[diabetes]] and all [[cancer]]s combined.<ref name="ABAM" /> These costs arise from the direct adverse effects of drugs and associated healthcare costs (e.g., [[emergency medical services]] and [[Patient#Outpatients and inpatients|outpatient and inpatient care]]), [[Sequela|long-term complications]] (e.g., [[lung cancer]] from smoking [[tobacco products]], [[liver cirrhosis]] and [[Alcohol-related dementia|dementia]] from chronic [[alcohol (drug)|alcohol]] consumption, and [[meth mouth]] from [[methamphetamine]] use), the loss of productivity and associated [[welfare]] costs, fatal and non-fatal [[accident]]s (e.g., [[traffic collision]]s), suicides, homicides, and incarceration, among others.<ref name="Societal toll"/><ref name="Epidem" /><ref name="ABAM" /><ref name="INCB 2013">{{cite book |title=International Narcotics Control Board Report: 2013 | date=2013 | publisher=United Nations – [[International Narcotics Control Board]] | isbn=978-92-1-148274-4 | url=https://www.incb.org/documents/Publications/AnnualReports/AR2013/English/AR_2013_E.pdf | accessdate=28 September 2018 | chapter-url=https://www.incb.org/documents/Publications/AnnualReports/Thematic_chapters/English/AR_2013_E_Chapter_I.pdf | chapter=Economic consequences of drug abuse}}</ref>
| |
| − | | |
| − | Examples of drug and behavioral addictions include [[alcoholism]], [[cannabis use disorder|marijuana addiction]], [[amphetamine addiction]], [[cocaine addiction]], [[nicotine#Reinforcement disorders|nicotine addiction]], [[opioid use disorder|opioid addiction]], [[food addiction]], [[video game addiction]], [[gambling addiction]], and [[sexual addiction]]. The only behavioral addiction recognized by the [[DSM-5]] and the [[ICD-10]] is gambling addiction. With the introduction of the ICD-11 gaming addiction was appended.<ref>{{Cite web|url=https://www.psychiatrictimes.com/article/gaming-addiction-icd-11-issues-and-implications|title=Gaming Addiction in ICD-11: Issues and Implications|last=Meredith E. Gansner|first=M. D.|date=2019-09-12|website=Psychiatric Times|language=en|access-date=2020-03-03}}</ref> The term ''addiction'' is misused frequently to refer to other compulsive behaviors or disorders, particularly ''[[substance dependence|dependence]]'', in news media.<ref name="Addiction-dependence distinction">{{cite web|author=American Psychiatric Association |title=Substance-Related and Addictive Disorders |url=http://www.dsm5.org/documents/substance%20use%20disorder%20fact%20sheet.pdf |publisher=American Psychiatric Publishing |accessdate=10 July 2015 |pages=1–2 |date=2013 |quote=Additionally, the diagnosis of dependence caused much confusion. Most people link dependence with "addiction" when in fact dependence can be a normal body response to a substance. |url-status=dead |archiveurl=https://web.archive.org/web/20150815050402/http://www.dsm5.org/Documents/Substance%20Use%20Disorder%20Fact%20Sheet.pdf |archivedate=15 August 2015 }}</ref> An important distinction between drug addiction and dependence is that drug dependence is a disorder in which cessation of drug use results in an unpleasant state of [[drug withdrawal|withdrawal]], which can lead to further drug use.<ref name="NHMH_3e terms-DSM flaw" /> Addiction is the compulsive use of a substance or performance of a behavior that is independent of withdrawal. Addiction can occur in the absence of dependence, and dependence can occur in the absence of addiction, although the two often occur together.
| |
| | | | |
| | ==Types of addiction== | | ==Types of addiction== |
| | {{addiction glossary}} | | {{addiction glossary}} |
| | + | Addiction has traditionally been used in reference to [[substance abuse]] where there are obvious physical dependencies. However, the term has been expanded to include behaviors that may lead to a reward (such as [[gambling]], eating, sexual activity, or even shopping).<ref>Constance Holden, [https://science.sciencemag.org/content/294/5544/980 'Behavioral' Addictions: Do They Exist?] ''Science'' 294(5544) (2001): 980–982. Retrieved August 31, 2022.</ref> A [[gene transcription factor]] known as [[ΔFosB]] has been identified as a necessary common factor involved in both behavioral and drug addictions, which are associated with the same set of neural adaptations in the [[reward system]].<ref name=Robison> Alfred J. Robison and Eric J. Nestler, [https://pubmed.ncbi.nlm.nih.gov/21989194/ Transcriptional and Epigenetic Mechanisms of Addiction] ''Nature Reviews Neuroscience'' 12(11) (2011):623-637. Retrieved August 31, 2022. </ref><ref name="ΔFosB reward">Kenneth Blum et al., [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4040958/ Sex, Drugs, and Rock ‘N’ Roll: Hypothesizing Common Mesolimbic Activation as a Function of Reward Gene Polymorphisms] ''Psychoactive Drugs'' 44(1) (2012):38–55. Retrieved August 31, 2022.</ref> |
| | | | |
| | + | Examples of drug and behavioral addictions include [[alcoholism]], [[cannabis use disorder|marijuana addiction]], [[amphetamine]] addiction, [[cocaine]] addiction, [[nicotine]] addiction, [[opioid]] addiction, [[food addiction]], [[video game]] addiction, [[gambling]] addiction, and [[sexual addiction]]. The only behavioral addiction recognized by the [[DSM-5]] and the [[ICD-10]] is gambling addiction. With the introduction of the ICD-11 gaming addiction was appended.<ref>Meredith E. Gansner, [https://www.psychiatrictimes.com/view/gaming-addiction-icd-11-issues-and-implications Gaming Addiction in ICD-11: Issues and Implications] ''Psychiatric Times'', September 12, 2019. Retrieved August 31, 2022.</ref> |
| | | | |
| − | Addiction canonically refers to substance abuse; however, the term connotation has been expanded to include behaviors that may lead to a reward (e.g., gambling, eating, or shopping)<ref>{{Cite journal|last=Holden|first=Constance|date=2001-11-02|title='Behavioral' Addictions: Do They Exist?|journal=Science|language=en|volume=294|issue=5544|pages=980–982|doi=10.1126/science.294.5544.980|issn=0036-8075|pmid=11691967}}</ref> since the 1990s. A [[gene transcription factor]] known as [[ΔFosB]] has been identified as a necessary common factor involved in both behavioral and drug addictions, which are associated with the same set of neural adaptations in the [[reward system]].<ref name="Nestler" /><ref name="Natural and drug addictions" /><ref name="ΔFosB reward">{{cite journal|year=2012|title=Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms|journal=Journal of Psychoactive Drugs|volume=44|issue=1|pages=38–55|doi=10.1080/02791072.2012.662112|pmc=4040958|pmid=22641964|quote=It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry.|vauthors=Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M}}</ref>
| + | The term ''addiction'' is misused frequently to refer to other compulsive behaviors or disorders, particularly ''[[substance dependence|dependence]]''.<ref>[https://mentalhealthgateway.org/substance-related-and-addictive-disorders/ Substance-Related and Addictive Disorders] ''Mental Health Gateway''. Retrieved August 31, 2022.</ref> Substance dependence is an adaptive state that develops from repeated drug administration, and which results in withdrawal (a set of unpleasant physical symptoms that are opposite of the effects of the drug) upon cessation of use. Addiction is compulsive, out-of-control use of a substance or performance of a behavior despite negative consequences. Addiction can occur in the absence of dependence, and dependence can occur in the absence of addiction, although the two often occur together. |
| | | | |
| | + | ==Biological mechanisms== |
| | + | [[ΔFosB]], a [[gene transcription]] factor, has been identified as playing a critical role in the development of addictive states in both behavioral addictions and drug addictions.<ref name=Robison/><ref name="Natural and drug addictions" /><ref name="ΔFosB reward" /> Overexpression of ΔFosB in the [[nucleus accumbens]] is [[necessary and sufficient]] for many of the [[neuroplasticity|neural adaptations]] seen in drug addiction; it has been implicated in addictions to [[alcoholism|alcohol]], [[cannabinoid]]s, [[cocaine]], [[nicotine]], [[phenylcyclidine]], and [[substituted amphetamines]]<ref name=Robison/><ref>Steven E. Hyman, Robert C. Malenka, and Eric J. Nestler, [https://www.semanticscholar.org/paper/Neural-mechanisms-of-addiction%3A-the-role-of-and-Hyman-Malenka/2abd7de87ac67b33317eb314716049b7088523c7 Neural mechanisms of addiction: the role of reward-related learning and memory] ''Annual Review of Neuroscience'' 29 (2006):565–598. Retrieved August 31, 2022.</ref> as well as addictions to natural rewards such as sex, exercise, and food.<ref name="Natural and drug addictions" /><ref name="ΔFosB reward" /> |
| | | | |
| − | ==Behavioral addiction==
| + | In the [[nucleus accumbens]], ΔFosB functions as a "sustained molecular switch" and "master control protein" in the development of an addiction. In other words, once "turned on" (sufficiently overexpressed) ΔFosB triggers a series of [[gene transcription|transcription]] events that ultimately produce an addictive state (compulsive reward-seeking involving a particular stimulus); this state is sustained for months after cessation of drug use due to the abnormal and exceptionally long [[half-life]] of ΔFosB isoforms.<ref> E.J. Nestler, M. Barrot, and D.W. Self, [https://pubmed.ncbi.nlm.nih.gov/11572966/ DeltaFosB: A Sustained Molecular Switch for Addiction] ''Proc Natl Acad Sci U S A'' 98(20) (2001):11042-22046. Retrieved August 31, 2022. </ref> ΔFosB expression in [[D1-type]] nucleus accumbens [[medium spiny neuron]]s directly and positively regulates drug [[self-administration]] and [[reward sensitization]] through [[positive reinforcement]] while decreasing sensitivity to [[aversion]].<ref name="Cellular basis" /> |
| − | '''Behavioral addiction'''{{#tag:ref|Synonyms of behavioral addiction include: '''process addiction''' and '''non-[[Chemical substance|substance]]-related addiction'''.<ref name="pmid19742294">{{cite journal |vauthors=Albrecht U, Kirschner NE, Grüsser SM |title=Diagnostic instruments for behavioural addiction: an overview |journal=Psychosom Med |volume=4 |issue= |pages=Doc11 |year=2007 |pmid=19742294 |pmc=2736529 |doi= |url=http://www.egms.de/static/en/journals/psm/2007-4/psm000043.shtml }}</ref><ref name="pmid16930171">{{cite journal |author=Potenza MN |title=Should addictive disorders include non-substance-related conditions? |journal=Addiction |volume=101 Suppl 1 |issue= |pages=142–51 |date=September 2006 |pmid=16930171 |doi=10.1111/j.1360-0443.2006.01591.x|url=https://semanticscholar.org/paper/f39e707a7f398d1840f772922fe1f713d8b6fde9 }}</ref><ref>{{cite journal |last1=Shaffer |first1=Howard J. |title=Understanding the means and objects of addiction: Technology, the internet, and gambling |journal=Journal of Gambling Studies |volume=12 |issue=4 |pages=461–9 |doi=10.1007/BF01539189 |pmid=24234163 |year=1996}}</ref>|group=note}} is a form of [[addiction]] that involves a [[compulsive behavior|compulsion]] to engage in a rewarding non-[[Chemical substance|substance]]-related behavior – sometimes called a '''''natural reward'''''<!--Phrase redirects here; bolded per MOS:BOLD—><ref name="Nestler">{{cite journal|date=November 2011|title=Transcriptional and epigenetic mechanisms of addiction|journal=Nat. Rev. Neurosci.|volume=12|issue=11|pages=623–637|doi=10.1038/nrn3111|pmc=3272277|pmid=21989194|quote=ΔFosB has been linked directly to several [[subtstance]]-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states.|vauthors=Robison AJ, Nestler EJ}}</ref><ref name="Natural and drug addictions">{{cite journal|author=Olsen CM|date=December 2011|title=Natural rewards, neuroplasticity, and non-drug addictions|url=|journal=Neuropharmacology|volume=61|issue=7|pages=1109–22|doi=10.1016/j.neuropharm.2011.03.010|pmc=3139704|pmid=21459101}}</ref> – despite any negative consequences to the person's physical, mental, social or financial well-being.<ref name="SteinHollander2009">{{cite book |first1=Dan J. |last1=Stein |first2=Eric |last2=Hollander |first3=Barbara Olasov |last3=Rothbaum |title=Textbook of Anxiety Disorders |url=https://books.google.com/books?id=quQY1R8vsZcC&pg=PA359 |accessdate=24 April 2010 |date=31 August 2009 |publisher=American Psychiatric Pub |isbn=978-1-58562-254-2|pages=359–}}</ref>
| |
| − | An addictive behavior is a behavior, or a stimulus related to a behavior (e.g., sex or food), that is both rewarding and reinforcing, and is associated with the development of an addiction. Addictions involving addictive behaviors are normally referred to as behavioral addictions.
| |
| | | | |
| − | The term ''behavioral addiction'' refers to a [[compulsive behavior|compulsion]] to engage in a [[natural reward]] – which is a behavior that is inherently rewarding (i.e., desirable or appealing) – despite adverse consequences.<ref name="NHM addiction-reward-reinforcement">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 364–65, 375 | edition = 2nd | chapter = Chapter 15: Reinforcement and Addictive Disorders | quote= The defining feature of addiction is compulsive, out-of-control drug use, despite negative consequences. ...<br />compulsive eating, shopping, gambling, and sex – so-called "natural addictions" – Indeed, addiction to both drugs and behavioral rewards may arise from similar dysregulation of the mesolimbic dopamine system.}}</ref><ref name="Natural and drug addictions" /><ref name="Nestler" /> Preclinical evidence has demonstrated that marked increases in the expression of ΔFosB through repetitive and excessive exposure to a natural reward induces the same behavioral effects and [[neuroplasticity]] as occurs in a drug addiction.<ref name="Natural and drug addictions" /><ref name="Systematic review - yet another DSM fail" /><ref name="Amph-Sex X-sensitization through D1 signaling" /><ref name="Amph-Sex X-sensitization through NMDA signaling" />
| + | Besides increased ΔFosB expression in the nucleus accumbens, there are many other correlations in the neurobiology of behavioral addictions with drug addictions. |
| | | | |
| − | Reviews of both clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form of [[sexual intercourse]] – as an addiction (i.e., [[sexual addiction]]).<ref name="Natural and drug addictions" /><ref name="Systematic review - yet another DSM fail" /> Moreover, [[#Reward sensitization|reward cross-sensitization]] between [[amphetamine]] and sexual activity, meaning that exposure to one increases the desire for both, has been shown to occur preclinically and clinically as a [[dopamine dysregulation syndrome]];<ref name="Natural and drug addictions" /><ref name="Systematic review - yet another DSM fail" /><ref name="Amph-Sex X-sensitization through D1 signaling" /><ref name="Amph-Sex X-sensitization through NMDA signaling" /> ΔFosB [[gene expression|expression]] is required for this cross-sensitization effect, which intensifies with the level of ΔFosB expression.<ref name="Natural and drug addictions" /><ref name="Amph-Sex X-sensitization through D1 signaling" /><ref name="Amph-Sex X-sensitization through NMDA signaling" />
| + | Behaviors like [[gambling]] have been linked to the brain's capacity to anticipate rewards. The reward system can be triggered by early detectors of the behavior, and trigger [[dopamine]] neurons to begin stimulating behaviors. But in some cases, it can lead to many issues due to error, or reward-prediction errors. These errors can act as teaching signals to create a complex behavior task over time.<ref name="dichiara" /> |
| | | | |
| − | Reviews of preclinical studies indicate that long-term frequent and excessive consumption of high fat or sugar foods can produce an addiction ([[food addiction]]).<ref name="Natural and drug addictions" /><ref name="Nestler" />
| + | One of the most important discoveries of addictions has been the drug based reinforcement and, even more important, reward based learning processes. Several structures of the brain are important in the conditioning process of behavioral addiction; these subcortical structures form the brain regions known as the [[reward system]]. One of the major areas of study is the [[amygdala]], a brain structure which involves emotional significance and associated learning. Research shows that dopaminergic projections from the [[ventral tegmental area]] facilitate a motivational or learned association to a specific behavior.<ref>Judson A. Brewer and Marc N. Potenza, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2222549/ The neurobiology and genetics of impulse control disorders: Relationships to drug addictions] ''Biochemical Pharmacology'' 75(1) (2008):63–75. Retrieved August 31, 2022.</ref> Dopamine neurons take a role in the learning and sustaining of many acquired behaviors. The most common mechanism of dopamine is to create addictive properties along with certain behaviors.<ref>Jean-Antoine Girault and Paul Greengard, [https://jamanetwork.com/journals/jamaneurology/fullarticle/785704 The Neurobiology of Dopamine Signaling] ''Archives of Neurology'' 61(5) (2004):641–644. Retrieved August 31, 2022.</ref> |
| | | | |
| − | Gambling provides a natural reward which is associated with compulsive behavior and for which clinical diagnostic manuals, namely the [[DSM-5]], have identified diagnostic criteria for an "addiction".<ref name="Natural and drug addictions" /> In order for a person's gambling behavior to meet criteria of an addiction, it shows certain characteristics, such as mood modification, compulsivity, and withdrawal. There is evidence from functional neuroimaging that gambling activates the reward system and the [[mesolimbic pathway]] in particular.<ref name="Natural and drug addictions" /><ref name="Behavioral addictions" /> Similarly, shopping and playing video games are associated with compulsive behaviors in humans and have also been shown to activate the mesolimbic pathway and other parts of the reward system.<ref name="Natural and drug addictions" /> Based upon this evidence, [[gambling addiction]], [[video game addiction]], and [[shopping addiction]] are classified accordingly.<ref name="Natural and drug addictions" /><ref name="Behavioral addictions" />
| + | There are three stages to the dopamine reward system: bursts of dopamine, triggering of behavior, and further impact to the behavior. Once electronically signaled, possibly through the behavior, dopamine neurons let out a ‘burst-fire’ of elements to stimulate areas along fast transmitting pathways. The behavior response then perpetuates the striated neurons to further send stimuli.<ref name="dichiara">Gaetano Di Chiara and Valentina Bassareo, [https://pubmed.ncbi.nlm.nih.gov/17174602/ Reward System and Addiction: What Dopamine Does and Doesn't Do] ''Current Opinion in Pharmacology'' 7(1) (2007):69–76. Retrieved August 31, 2022.</ref> Once the behavior is triggered, it is difficult to work away from the dopamine reward system. |
| | | | |
| − | ===Psychiatric and medical classifications=== | + | ==Substance use disorder== |
| − | Diagnostic models do not currently include the criteria necessary to identify behaviors as addictions in a clinical setting. Behavioral addictions have been proposed as a new class in [[DSM-5]], but the only category included is gambling addiction. Internet gaming addiction is included in the appendix as a condition for further study.<ref>{{cite journal |last1=Kuss|first1=Daria|title=Internet gaming addiction: current perspectives|journal= Psychology Research and Behavior Management|issue=6 |pages=125–137 |doi=10.2147/PRBM.S39476 |year=2013 |pmc=3832462 |pmid=24255603 |volume=6}}</ref><ref>{{cite web|last1=Shenfield|first1=Tali|title=Is your child a gaming addict?|url=http://www.psy-ed.com/wpblog/child-gaming-addiction/|website=Advanced Psychology}}</ref>
| + | [[Substance use disorder]] ('''SUD'''), also known as a '''drug use disorder''', is the persistent use of drugs (including alcohol) despite substantial harm and adverse consequences. Such addiction can be defined as "the compulsive seeking and taking of drugs despite horrendous consequences or loss of control over drug use."<ref name="Cellular basis">Eric J. Nestler, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3898681/ Cellular basis of memory for addiction] ''Dialogues Clin Neurosci'' 15(4) (2013): 431–443. Retrieved August 31, 2022.</ref> Substance use disorders are characterized by an array of mental, physical, and behavioral symptoms that may cause problems related to loss of control, strain to one's interpersonal life, hazardous use, tolerance, and withdrawal.<ref name=DSM-5>American Psychiatric Association, ''Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5'' (American Psychiatric Publishing, 2013, ISBN 978-0890425558).</ref> |
| | | | |
| − | Behavioral addictions, which are sometimes referred to as impulse control disorders, are increasingly recognized as treatable forms of addiction.<ref>Grant, Jon: Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions</ref>
| + | In the 5th edition of the [[Diagnostic and Statistical Manual of Mental Disorders]] ([[DSM-5]]), [[substance abuse]] and [[substance dependence]] were merged into the category of substance use disorders.ref name=DSM-5/> The severity of substance use disorders can vary widely; in the diagnosis of a SUD, the severity of an individual's SUD is qualified as ''mild'', ''moderate'', or ''severe'' on the basis of how many of the [[#Diagnosis|11 diagnostic criteria]] are met. |
| − | The type of excessive behaviors identified as being addictive include [[Problem gambling|gambling]], [[eating disorder|food]], [[Chocoholic|chocolate]], [[sexual addiction|sexual intercourse]], use of [[pornography addiction|pornography]], use of [[computer addiction|computers]], playing [[Video game addiction|video games]], use of the [[Internet addiction disorder|internet]] and [[Digital media use and mental health|other digital media]], [[Physical exercise#Excessive exercise|exercise]], and [[Compulsive buying disorder|shopping]].
| |
| | | | |
| − | Researching [[Food addiction|addiction to food]], for example, a 2009 Scripps Research Institute study found evidence that the same molecular mechanisms correlated with human drug addiction also exist in compulsive overeating in obese rats. The dopamine D<sub>2</sub> receptor studied is associated with vulnerability to drug addiction in humans. It was found downregulated in obese rats exposed to a high fat diet, and further reductions of the receptor increased compulsive eating. The D<sub>2</sub> receptor responds to dopamine, a central neurotransmitter released in anticipation of rewarding, satiating experiences such as those involving food, sex or psychoactive drugs.<ref>{{cite journal |doi=10.1038/nn.2519 |lay-url=https://www.sciencedaily.com/releases/2010/03/100328170243.htm |laysource=ScienceDaily |laydate=29 March 2010 |title=Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats |year=2010 |last1=Johnson |first1=Paul M |last2=Kenny |first2=Paul J |journal=Nature Neuroscience |volume=13 |issue=5 |pages=635–41 |pmid=20348917 |pmc=2947358}}</ref>
| + | Drug classes that are involved in SUD include: [[Alcoholism|alcohol]]; [[Caffeine dependence|caffeine]]; [[Cannabis (drug)|cannabis]]; hallucinogens (such as [[arylcyclohexylamine]]s); other hallucinogens (such as [[Lysergic acid diethylamide|LSD]]); [[inhalant]]s; [[Opioid use disorder|opioids]]; [[sedative]]s, [[hypnotic]]s, or [[anxiolytic]]s; [[stimulant]]s; [[Tobacco smoking|tobacco]]; and other or unknown substances.<ref>Substance Abuse and Mental Health Services Administration, [https://www.ncbi.nlm.nih.gov/books/NBK519702/ Substance Use Disorders] ''Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health'', 2016. Retrieved August 31, 2022.</ref> |
| | | | |
| − | In August 2011, the [[American Society of Addiction Medicine]] (ASAM) issued a public statement defining all addiction in terms of brain changes. "Addiction is a primary, chronic disease of brain reward, motivation, memory and related circuitry."<ref>American Society of Addiction Medicine. Public Policy Statement: Definition of Addiction. https://www.asam.org/resources/definition-of-addiction</ref> | + | Addiction exacts an "astoundingly high financial and human toll" on individuals and society as a whole.<ref name=Nestleretal/> In the United States, the total economic cost to society is greater than that of all types of [[diabetes]] and all [[cancer]]s combined: |
| | + | <blockquote>Risky substance use and untreated addiction account for one-third of inpatient hospital costs and 20 percent of all deaths in the United States each year, and cause or contribute to more than 100 other conditions requiring medical care, as well as vehicular crashes, other fatal and non-fatal injuries, overdose deaths, suicides, homicides, domestic discord, the highest incarceration rate in the world and many other costly social consequences. The economic cost to society is greater than the cost of diabetes and all cancers combined.<ref name="ABAM">Dennis Tartaglia, [https://www.abms.org/news-events/abms-officially-recognizes-addiction-medicine-as-a-subspecialty/ ABMS Officially Recognizes Addiction Medicine as a Subspecialty] ''American Board of Addiction Medicine'', March 14, 2016. Retrieved August 31, 2022.</ref></blockquote> |
| | | | |
| − | The following excerpts are taken from the organization's FAQs: <blockquote>The new ASAM definition makes a departure from equating addiction with just [[substance dependence]], by describing how addiction is also related to behaviors that are rewarding. This is the first time that ASAM has taken an official position that addiction is not solely "substance dependence." This definition says that addiction is about functioning and brain circuitry and how the structure and function of the brains of persons with addiction differ from the structure and function of the brains of persons who do not have addiction. It talks about reward circuitry in the brain and related circuitry, but the emphasis is not on the external rewards that act on the reward system. Food and sexual behaviors and gambling behaviors can be associated with the "pathological pursuit of rewards" described in this new definition of addiction. </blockquote>
| + | These costs arise from the direct adverse effects of drugs and associated [[healthcare]] costs, [[Sequela|long-term complications]] (such as [[lung cancer]] from smoking [[tobacco products]], [[liver cirrhosis]] and [[Alcohol-related dementia|dementia]] from chronic [[alcohol (drug)|alcohol]] consumption, and [[meth mouth]] from [[methamphetamine]] use), the loss of productivity and associated [[welfare]] costs, fatal and non-fatal [[accident]]s, [[suicide]]s, [[homicide]]s, and incarceration, among others.<ref>United Nations Office on Drugs and Crime, ''International Narcotics Control Board Report: 2013'' (United Nations, 2014, ISBN 978-9211482744).</ref> |
| | | | |
| − | <blockquote>We all have the brain reward circuitry that makes food and sex rewarding. In fact, this is a survival mechanism. In a healthy brain, these rewards have feedback mechanisms for satiety or 'enough.' In someone with addiction, the circuitry becomes dysfunctional such that the message to the individual becomes ‘more’, which leads to the pathological pursuit of rewards and/or relief through the use of substances and behaviors. So, anyone who has addiction is vulnerable to food and sex addiction.</blockquote> | + | ===Diagnosis=== |
| | + | Diagnosis of [[substance use disorder]] (SUD) usually involves an in-depth examination, typically by psychiatrist, psychologist, or drug and alcohol counselor.<ref>[https://www.mayoclinic.org/diseases-conditions/drug-addiction/symptoms-causes/syc-20365112 Drug addiction (substance use disorder)] ''Mayo Clinic''. Retrieved August 31, 2022.</ref> The most commonly used guidelines are published in the ''Diagnostic and Statistical Manual of Mental Disorders'' (DSM-5).<ref name=DSM-5/> |
| | | | |
| − | Since ASAM released its statement, and shortly before its release, additional new studies have come out on Internet addiction. They reveal the same fundamental brain changes seen in other addicts of drugs.<ref>{{cite journal |doi=10.1371/journal.pone.0030253 |title=Abnormal White Matter Integrity in Adolescents with Internet Addiction Disorder: A Tract-Based Spatial Statistics Study |year=2012 |editor1-last=Frasch |editor1-first=Martin Gerbert |last1=Lin |first1=Fuchun |last2=Zhou |first2=Yan |last3=Du |first3=Yasong |last4=Qin |first4=Lindi |last5=Zhao |first5=Zhimin |last6=Xu |first6=Jianrong |last7=Lei |first7=Hao |journal=PLoS ONE |volume=7 |pages=e30253 |pmid=22253926 |issue=1 |pmc=3256221|bibcode=2012PLoSO...730253L }}</ref><ref>{{cite journal |doi=10.1016/j.jpsychires.2011.06.017 |title=Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: An fMRI study during a guessing task |year=2011 |last1=Dong |first1=Guangheng |last2=Huang |first2=Jie |last3=Du |first3=Xiaoxia |journal=Journal of Psychiatric Research |volume=45 |issue=11 |pages=1525–9 |pmid=21764067}}</ref><ref>{{cite journal |doi=10.1016/j.neulet.2011.05.047 |title=Male Internet addicts show impaired executive control ability: Evidence from a color-word Stroop task |year=2011 |last1=Dong |first1=Guangheng |last2=Zhou |first2=Hui |last3=Zhao |first3=Xuan |journal=Neuroscience Letters |volume=499 |issue=2 |pages=114–8 |pmid=21645588 }}</ref><ref>{{cite journal |doi=10.1371/journal.pone.0020708 |title=Microstructure Abnormalities in Adolescents with Internet Addiction Disorder |year=2011 |editor1-last=Yang |editor1-first=Shaolin |last1=Yuan |first1=Kai |last2=Qin |first2=Wei |last3=Wang |first3=Guihong |last4=Zeng |first4=Fang |last5=Zhao |first5=Liyan |last6=Yang |first6=Xuejuan |last7=Liu |first7=Peng |last8=Liu |first8=Jixin |last9=Sun |first9=Jinbo |last10=von Deneen |first10=K. M. |last11=Gong |first11=Q |last12=Liu |first12=Y |last13=Tian |first13=J |journal=PLoS ONE |volume=6 |issue=6 |pages=e20708 |pmid=21677775 |pmc=3108989|display-authors=8 |bibcode=2011PLoSO...620708Y }}</ref><ref>{{cite journal |doi=10.1097/WNR.0b013e328346e16e |title=Reduced striatal dopamine D2 receptors in people with Internet addiction |year=2011 |last1=Kim |first1=Sang Hee |last2=Baik |first2=Sang-Hyun |last3=Park |first3=Chang Soo |last4=Kim |first4=Su Jin |last5=Choi |first5=Sung Won |last6=Kim |first6=Sang Eun |journal=NeuroReport |volume=22 |issue=8 |pages=407–11 |pmid=21499141}}</ref><ref>{{cite journal |pmid=21937800 |url=http://xbyx.xysm.net/xbwk/fileup/PDF/201108744.pdf |year=2011 |last1=Du |first1=W |title=Functional magnetic resonance imaging of brain of college students with internet addiction |last2=Liu |first2=J |last3=Gao |first3=X |last4=Li |first4=L |last5=Li |first5=W |last6=Li |first6=X |last7=Zhang |first7=Y |last8=Zhou |first8=S |script-title=zh:网络成瘾大学生脑功能性磁共振成像特点 |trans-title=Functional magnetic resonance imaging of brain of college students with internet addiction |language=Chinese |volume=36 |issue=8 |pages=744–9 |doi=10.3969/j.issn.1672-7347.2011.08.008 |journal=中南大学学报 (医学版) [Journal of Central South University (Medical sciences)] |access-date=18 July 2013 |archive-url=https://web.archive.org/web/20171210123624/http://xbyx.xysm.net/xbwk/fileup/PDF/201108744.pdf |archive-date=10 December 2017 |url-status=dead |df=dmy-all }}</ref> Another 2011 study found that the risk of Internet addiction in men was about three times more than women. Researchers noted, <blockquote>Internet addiction is a psychosocial disorder and its characteristics are as follows: [[Drug tolerance|tolerance]], [[Drug withdrawal|withdrawal symptoms]], affective disorders, and problems in social relations. Internet usage creates psychological, social, school and/or work difficulties in a person's life. Eighteen percent of study participants were considered to be pathological Internet users, whose excessive use of the Internet was causing academic, social, and interpersonal problems. Excessive Internet use may create a heightened level of psychological arousal, resulting in little sleep, failure to eat for long periods, and limited physical activity, possibly leading to the user experiencing physical and mental health problems such as depression, OCD, low family relationships and anxiety.<ref>{{cite journal |pmid=22091309 |year=2011 |last1=Alavi |first1=SS |last2=Maracy |first2=MR |last3=Jannatifard |first3=F |last4=Eslami |first4=M |title=The effect of psychiatric symptoms on the internet addiction disorder in Isfahan's University students |volume=16 |issue=6 |pages=793–800 |pmc=3214398 |journal=Journal of Research in Medical Sciences}}</ref></blockquote>
| + | The 5th edition of the [[Diagnostic and Statistical Manual of Mental Disorders]] (DSM-5) uses the term "[[substance use disorder]]" to refer to a spectrum of drug use-related disorders. The DSM-5 eliminates the terms "[[drug abuse|abuse]]" and "dependence" from diagnostic categories, instead using the specifiers of ''mild'', ''moderate'' and ''severe'' to indicate the extent of disordered use. These specifiers are determined by the number of diagnostic criteria present in a given case. In the DSM-5, the term ''drug addiction'' is synonymous with ''severe substance use disorder''. |
| | | | |
| − | ===Treatment=== | + | There are 11 diagnostic criteria which can be broadly categorized into issues arising from substance use related to loss of control, strain to one's interpersonal life, hazardous use, and pharmacologic effects. DSM-5 guidelines for the diagnosis of a substance use disorder requires that the individual have significant impairment or distress from their pattern of drug use, and at least two of the symptoms listed below in a given year.<ref name=DSM-5/> |
| | | | |
| − | Behavioral addiction is a treatable condition. Treatment options include [[psychotherapy]] and [[psychopharmacology|psychopharmacotherapy]] (i.e., medications) or a combination of both. [[Cognitive behavioral therapy]] (CBT) is the most common form of psychotherapy used in treating behavioral addictions; it focuses on identifying patterns that trigger [[compulsive behavior]] and making lifestyle changes to promote healthier behaviors. Because cognitive behavioral therapy is considered a short term therapy, the number of sessions for treatment normally ranges from five to twenty. During the session, therapists will lead patients through the topics of identifying the issue, becoming aware of one's thoughts surround the issue, identifying any negative or false thinking, and reshaping said negative and false thinking. While CBT does not cure behavioral addiction, it does help with coping with the condition in a healthy way. Currently, there are no medications approved for treatment of behavioral addictions in general, but some medications used for treatment of drug addiction may also be beneficial with specific behavioral addictions.<ref name="Behavioral addictions">{{cite journal | vauthors = Grant JE, Potenza MN, Weinstein A, Gorelick DA | title = Introduction to behavioral addictions | journal = Am. J. Drug Alcohol Abuse | volume = 36 | issue = 5 | pages = 233–241 | date = September 2010 | pmid = 20560821 | pmc = 3164585 | doi = 10.3109/00952990.2010.491884 | quote = Naltrexone, a mu-opioid receptor antagonist approved by the US Food and Drug Administration for the treatment of alcoholism and opioid dependence, has shown efficacy in controlled clinical trials for the treatment of pathological gambling and kleptomania (76–79), and promise in uncontrolled studies of compulsive buying (80), compulsive sexual behavior (81), internet addiction (82), and pathologic skin picking (83). ... Topiramate, an anti-convulsant which blocks the AMPA subtype of glutamate receptor (among other actions), has shown promise in open-label studies of pathological gambling, compulsive buying, and compulsive skin picking (85), as well as efficacy in reducing alcohol (86), cigarette (87), and cocaine (88) use. N-acetyl cysteine, an amino acid that restores extracellular glutamate concentration in the nucleus accumbens, reduced gambling urges and behavior in one study of pathological gamblers (89), and reduces cocaine craving (90) and cocaine use (91) in cocaine addicts. These studies suggest that glutamatergic modulation of dopaminergic tone in the nucleus accumbens may be a mechanism common to behavioral addiction and substance use disorders (92).}}</ref> Any unrelated psychiatric disorders should be kept under control, and differentiated from the contributing factors that cause the addiction.
| + | # Using more of a substance than planned, or using a substance for a longer interval than desired |
| | + | # Inability to cut down despite desire to do so |
| | + | # Spending substantial amount of the day obtaining, using, or recovering from substance use |
| | + | # Cravings or intense urges to use |
| | + | # Repeated usage causes or contributes to an inability to meet important social, or professional obligations |
| | + | # Persistent usage despite user's knowledge that it is causing frequent problems at work, school, or home |
| | + | # Giving up or cutting back on important social, professional, or leisure activities because of use |
| | + | # Using in physically hazardous situations, or usage causing physical or mental harm |
| | + | # Persistent use despite the user's awareness that the substance is causing or at least worsening a physical or mental problem |
| | + | # Tolerance: needing to use increasing amounts of a substance to obtain its desired effects |
| | + | # Withdrawal: characteristic group of physical effects or symptoms that emerge as amount of substance in the body decreases |
| | | | |
| − | ===Research===
| + | ''Tolerance'' is the process by which the body continually adapts to the substance and requires increasingly larger amounts to achieve the original effects. [[Physical dependence]] occurs when the body has adjusted by incorporating the substance into its "normal" functioning – attained [[homeostasis]] – and therefore physical withdrawal symptoms occur upon cessation of use. Symptoms of ''withdrawal'' generally include, but are not limited to, body aches, [[anxiety]], [[irritability]], intense [[craving (withdrawal)|cravings]] for the substance, [[nausea]], [[hallucination]]s, [[headache]]s, cold sweats, [[tremor]]s, and seizures. |
| | | | |
| − | A recent narrative review<ref>{{Cite journal|last=Starcevic|first=Vladan|last2=Khazaal|first2=Yasser|date=2017-04-07|title=Relationships between Behavioural Addictions and Psychiatric Disorders: What Is Known and What Is Yet to Be Learned?|journal=Frontiers in Psychiatry|volume=8|pages=53|doi=10.3389/fpsyt.2017.00053|pmid=28439243|pmc=5383701|issn=1664-0640}}</ref> (2017) reviewed the existing literature for studies reporting associations between behavioural addictions (pathological gambling, problematic internet use, problematic online gaming, compulsive sexual behaviour disorder, compulsive buying and exercise addiction) and psychiatric disorders. Overall, there is solid evidence for associations between behavioural addictions and [[mood disorder]], [[anxiety disorder]] as well as [[substance use disorder]]s. Associations between [[ADHD]] may be specific to problematic internet use and problematic online gaming. The authors also conclude that most of current research on the association between behavioural addictions and psychiatric disorders has several limitations: they are mostly cross-sectional, are not from representative samples, and are often based on small samples, among others. Especially more longitudinal studies are needed to establish the direction of causation, i.e. whether behavioural addictions are a cause or a consequence of psychiatric disorders.
| + | There are additional qualifiers and exceptions outlined in the DSM. For instance, if an individual is taking [[opiate|opiates]] as prescribed, they may experience physiologic effects of tolerance and withdrawal, but this would not cause an individual to meet criteria for a SUD without additional symptoms also being present.<ref name=DSM-5/> |
| | | | |
| − | Another growing area is [[social media addiction]]. Psychology researchers surveyed 253 undergraduate students at the University of Albany and found that not only is social media (particularly Facebook) itself potentially addictive, those who use it may also be at greater risk for substance abuse.<ref>{{cite web |url=http://www.albany.edu/news/56604.php |title=Craving Facebook? UAlbany Study Finds Social Media to be Potentially Addictive, Associated with Substance Abuse }}</ref>
| + | Medical researchers who actively study addiction have criticized the DSM classification of addiction for being flawed and involving arbitrary diagnostic criteria.<ref name=Nestleretal/> Writing in 2013, Thomas Insel, the director of the United States National Institute of Mental Health discussed the invalidity of the DSM-5's classification of mental disorders: |
| | + | <blockquote>While DSM has been described as a "Bible" for the field, it is, at best, a dictionary, creating a set of labels and defining each. The strength of each of the editions of DSM has been "reliability" – each edition has ensured that clinicians use the same terms in the same ways. The weakness is its lack of validity. Unlike our definitions of ischemic heart disease, lymphoma, or AIDS, the DSM diagnoses are based on a consensus about clusters of clinical symptoms, not any objective laboratory measure. In the rest of medicine, this would be equivalent to creating diagnostic systems based on the nature of chest pain or the quality of fever.<ref>John Horgan, [https://blogs.scientificamerican.com/cross-check/psychiatry-in-crisis-mental-health-director-rejects-psychiatric-bible-and-replaces-with-nothing/ Psychiatry in Crisis! Mental Health Director Rejects Psychiatric "Bible" and Replaces With Nothing] ''Scientific American'', May 4, 2013. Retrieved August 31, 2022.</ref></blockquote> |
| | | | |
| − | ===Biomolecular mechanisms===
| + | Given that addiction manifests in structural changes to the brain, it is possible that non-invasive [[neuroimaging]] scans obtained via [[MRI]] could be used to help diagnose addiction in the future.<ref>William H. Hampton, Italia M. Hanik, and Ingrid R. Olson, [https://pubmed.ncbi.nlm.nih.gov/30875650/ Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research] ''Drug and Alcohol Dependence'' 197(4) (2019):288–298. Retrieved August 31, 2022.</ref> As a diagnostic [[biomarker (medicine)|biomarker]], [[ΔFosB]] expression could be used to diagnose addiction, but this would require a [[brain biopsy]] and therefore is not used in clinical practice. |
| − | {{main|ΔFosB}}
| |
| | | | |
| − | [[ΔFosB]], a [[gene transcription]] factor, has been identified as playing a critical role in the development of addictive states in both behavioral addictions and drug addictions.<ref name="Nestler" /><ref name="Natural and drug addictions" /><ref name="ΔFosB reward" /> Overexpression of ΔFosB in the [[nucleus accumbens]] is [[necessary and sufficient]] for many of the [[neuroplasticity|neural adaptations]] seen in drug addiction;<ref name="Nestler" /> it has been implicated in addictions to [[alcoholism|alcohol]], [[cannabinoid]]s, [[cocaine]], [[nicotine]], [[phenylcyclidine]], and [[substituted amphetamines]]<ref name="Nestler" /><ref name="Nestler, Hyman, and Malenka 2">{{cite journal|year=2006|title=Neural mechanisms of addiction: the role of reward-related learning and memory|url=https://semanticscholar.org/paper/2abd7de87ac67b33317eb314716049b7088523c7|journal=Annu. Rev. Neurosci.|volume=29|issue=|pages=565–598|doi=10.1146/annurev.neuro.29.051605.113009|pmid=16776597|vauthors=Hyman SE, Malenka RC, Nestler EJ}}</ref><ref name="Addiction genetics">{{cite journal|date=January 2013|title=Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants|url=|journal=Prog. Neurobiol.|volume=100|issue=|pages=60–80|doi=10.1016/j.pneurobio.2012.10.001|pmc=3525776|pmid=23085425|vauthors=Steiner H, Van Waes V}}</ref><ref name="Alcoholism ΔFosB">{{cite web|url=http://www.genome.jp/kegg-bin/show_pathway?hsa05034+2354|title=Alcoholism – Homo sapiens (human)|author=Kanehisa Laboratories|date=2 August 2013|website=KEGG Pathway|accessdate=10 April 2014}}</ref> as well as addictions to natural rewards such as sex, exercise, and food.<ref name="Natural and drug addictions" /><ref name="ΔFosB reward" /> A recent study also demonstrated a [[cross-sensitization]] between drug reward (amphetamine) and a natural reward (sex) that was mediated by ΔFosB.<ref name="Amph and sex addiction">{{cite journal|date=February 2013|title=Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator|journal=J. Neurosci.|volume=33|issue=8|pages=3434–42|doi=10.1523/JNEUROSCI.4881-12.2013|pmc=3865508|pmid=23426671|quote=Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets.|vauthors=Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM}}</ref>
| + | ===Treatment=== |
| | + | Treatment for substance abuse disorder is not simple. Rather than a single treatment, a variety of different approaches are required for success: |
| | + | <blockquote>In order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities.<ref name=Taylor/></blockquote> |
| | | | |
| − | Besides increased ΔFosB expression in the nucleus accumbens, there are many other correlations in the neurobiology of behavioral addictions with drug addictions.
| + | ====Detoxification==== |
| | + | Depending on the severity of use, and the given substance, early treatment of acute withdrawal may include medical [[Drug detoxification|detoxification]]. Of note, acute withdrawal from heavy [[alcohol]] use must be done under medical supervision to prevent a potentially deadly withdrawal syndrome known as [[delirium tremens]]. |
| | | | |
| − | One of the most important discoveries of addictions has been the drug based reinforcement and, even more important, reward based learning processes. Several structures of the brain are important in the conditioning process of behavioral addiction; these subcortical structures form the brain regions known as the [[reward system]]. One of the major areas of study is the [[amygdala]], a brain structure which involves emotional significance and associated learning. Research shows that dopaminergic projections from the [[ventral tegmental area]] facilitate a motivational or learned association to a specific behavior.<ref>{{cite journal|last1=Brewer|first1=Judson A.|last2=Potenza|first2=Marc N.|year=2008|title=The neurobiology and genetics of impulse control disorders: Relationships to drug addictions|journal=Biochemical Pharmacology|volume=75|issue=1|pages=63–75|doi=10.1016/j.bcp.2007.06.043|pmc=2222549|pmid=17719013}}</ref>
| + | ====Therapy==== |
| − | Dopamine neurons take a role in the learning and sustaining of many acquired behaviors. Research specific to Parkinson's disease has led to identifying the intracellular signaling pathways that underlie the immediate actions of dopamine. The most common mechanism of dopamine is to create addictive properties along with certain behaviors.<ref>{{cite journal|last1=Girault|first1=Jean-Antoine|last2=Greengard|first2=P|year=2004|title=The Neurobiology of Dopamine Signaling|journal=Archives of Neurology|volume=61|issue=5|pages=641–4|doi=10.1001/archneur.61.5.641|pmid=15148138|doi-access=free}}</ref> There are three stages to the dopamine reward system: bursts of dopamine, triggering of behavior, and further impact to the behavior. Once electronically signaled, possibly through the behavior, dopamine neurons let out a ‘burst-fire’ of elements to stimulate areas along fast transmitting pathways. The behavior response then perpetuates the striated neurons to further send stimuli. The fast firing of dopamine neurons can be monitored over time by evaluating the amount of extracellular concentrations of dopamine through micro dialysis and brain imaging. This monitoring can lead to a model in which one can see the multiplicity of triggering over a period of time.<ref name="dichiara">{{cite journal|last1=Dichiara|first1=G|last2=Bassareo|first2=V|year=2007|title=Reward system and addiction: What dopamine does and doesn't do|journal=Current Opinion in Pharmacology|volume=7|issue=1|pages=69–76|doi=10.1016/j.coph.2006.11.003|pmid=17174602}}</ref> Once the behavior is triggered, it is hard to work away from the dopamine reward system.
| + | [[File:Cognitive behavioral therapy - basic tenets.png|thumb|350px|Cognitive Behavioral Therapy (CBT) has been found moderately effective in treating addictions]] |
| | + | Therapeutic treatments usually involve planning for specific ways to avoid the addictive stimulus, and therapeutic interventions intended to help a client learn healthier ways to find satisfaction. Therapists attempt to tailor intervention approaches to specific influences that affect addictive behavior, using therapeutic interviews in an effort to discover factors that led a person to embrace unhealthy, addictive sources of pleasure or relief from pain. |
| | | | |
| − | Behaviors like gambling have been linked to the new found idea of the brain's capacity to anticipate rewards. The reward system can be triggered by early detectors of the behavior, and trigger dopamine neurons to begin stimulating behaviors. But in some cases, it can lead to many issues due to error, or reward-prediction errors. These errors can act as teaching signals to create a complex behavior task over time.<ref name="dichiara" />
| + | A meta-analytic review on the efficacy of various [[Behavioral therapy|behavioral therapies]] for treating drug and behavioral addictions found that [[cognitive behavioral therapy]] (such as [[relapse prevention]] and [[contingency management]]), [[motivational interviewing]], and a [[Community reinforcement approach and family training|community reinforcement approach]] were effective interventions with moderate effect sizes.<ref>M. Walter, et al., [https://pubmed.ncbi.nlm.nih.gov/25893493/ Psychosocial Treatment of Addictive Disorders – An Overview of Psychotherapeutic Options and their Efficacy] ''Fortschr Neurol Psychiatr'' 83(4) (2015):201–210. Retrieved August 31, 2022.</ref> |
| | | | |
| − | ==Drug and alcohol addiction== | + | Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (such as [[marathon running]]), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and for psychostimulant addiction in particular.<ref name="Natural and drug addictions" /><ref name="Running vs addiction">Wendy J Lynch, Alexis B Peterson, Victoria Sanchez, Jean Abel, and Mark A. Smith, [https://pubmed.ncbi.nlm.nih.gov/23806439/ Exercise as a Novel Treatment for Drug Addiction: A Neurobiological and Stage-Dependent Hypothesis] ''Neurosci Biobehav Rev'' 37(8) (2013):1622–1644. Retrieved August 31, 2022.</ref> Consistent aerobic exercise reduces drug addiction risk, decreases drug self-administration, reduces the likelihood of relapse, and induces opposite effects on [[striatum|striatal]] [[dopamine receptor D2|dopamine receptor D<sub>2</sub>]] (DRD2) signaling (increased DRD2 density) to those induced by addictions to several drug classes (decreased DRD2 density). Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.<ref name="Natural and drug addictions" /><ref name="Running vs addiction" /> |
| | | | |
| − | ==Risk factors== | + | ==== Medication ==== |
| − | {{See|Addiction vulnerability}}
| + | [[Medication-assisted treatment]] (MAT) refers to the combination of behavioral interventions and medications to treat substance use disorders. Certain medications can be useful in treating severe substance use disorders. In the United States, several medications, such as disulfiram and methadone, are approved to treat alcohol and opioid use disorders.<ref>American Psychiatric Association, ''Practice Guidelines for the Treatment of Psychiatric Disorders'' (American Psychiatric Publishing, 2006, ISBN 978-0890423851).</ref> There are no approved medications for cocaine, methamphetamine, or other substance use disorders. |
| | | | |
| − | There are a number of genetic and environmental risk factors for developing an addiction, that vary across the population.<ref name="Cellular basis" /><ref name="Transgenerational epigenetic inheritance in addiction" /> Genetic and environmental risk factors each account for roughly half of an individual's risk for developing an addiction;<ref name="Cellular basis" /> the contribution from epigenetic risk factors to the total risk is unknown.<ref name="Transgenerational epigenetic inheritance in addiction" /> Even in individuals with a relatively low genetic risk, exposure to sufficiently high doses of an addictive drug for a long period of time (e.g., weeks–months) can result in an addiction.<ref name="Cellular basis" />
| + | Approved medications can be used as part of broader treatment plans to help a patient function comfortably without illicit opioids or alcohol.<ref>Antoine B. Douaihy, Thomas M. Kelly, and Carl Sullivan, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3767185/ Medications for Substance Use Disorders] ''Soc Work Public Health'' 28(0) (2013): 264–278. Retrieved August 31, 2022.</ref> Medications can be used in treatment to lessen withdrawal symptoms. Evidence has demonstrated the efficacy of MAT at reducing illicit drug use and overdose deaths, improving retention in treatment, and reducing HIV transmission.<ref> Office of the Surgeon General, ''Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health'' (CreateSpace, 2017, ISBN 978-1974580620).</ref> |
| | | | |
| − | === Genetic factors === | + | =====Alcohol addiction===== |
| − | {{See also|Alcoholism#Genetic variation}}
| + | [[Alcohol]], like opioids, can induce a severe state of [[physical dependence]] and produce withdrawal symptoms such as [[delirium tremens]]. Because of this, treatment for alcohol addiction usually involves a combined approach dealing with dependence and addiction simultaneously. Benzodiazepines have the largest and the best evidence base in the treatment of alcohol withdrawal and are considered the gold standard of [[alcohol detoxification]].<ref>Ankur Sachdeva, Mona Choudhary, and Mina Chandra, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4606320/ Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond] ''Journal of Clinical and Diagnostic Research'' 9(9) (2015):VE01–VE07. Retrieved August 31, 2022.</ref> |
| | | | |
| − | It has long been established that genetic factors along with environmental (e.g., psychosocial) factors are significant contributors to addiction vulnerability.<ref name="Cellular basis" /><ref name="Transgenerational epigenetic inheritance in addiction" /> [[Epidemiological method|Epidemiological]] studies estimate that genetic factors account for 40–60% of the risk factors for [[alcoholism]].<ref>Mayfield RD, Harris RA,1, Schuckit MA (May 2008) [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2442454/ "Genetic factors influencing alcohol dependence"] [[PMID]] [https://www.ncbi.nlm.nih.gov/pubmed/18362899 18362899]</ref> Similar rates of heritability for other types of drug addiction have been indicated by other studies.<ref name=Kendler94/> Knestler hypothesized in 1964 that a gene or group of genes might contribute to predisposition to addiction in several ways. For example, altered levels of a normal protein due to environmental factors could then change the structure or functioning of specific brain neurons during development. These altered brain neurons could change the susceptibility of an individual to an initial drug use experience. In support of this hypothesis, animal studies have shown that environmental factors such as stress can affect an animal's genotype.<ref name=Kendler94>{{cite journal |vauthors=Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ |title=A twin-family study of alcoholism in women |journal=Am J Psychiatry |volume=151 |issue=5 |pages=707–15 |date=May 1994 |pmid=8166312 |doi=10.1176/ajp.151.5.707 }}</ref>
| + | Pharmacological treatments for alcohol addiction include [[naltrexone]] (opioid antagonist), [[disulfiram]], [[acamprosate]], and [[topiramate]]. Rather than substituting for alcohol, these drugs are intended to affect the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.<ref>Bradford T. Winslow, Mary Onysko, and Melanie Hebert, [https://www.aafp.org/pubs/afp/issues/2016/0315/p457.html Medications for Alcohol Use Disorder] ''American Family Physician'' 93(6) (2016):457-465. Retrieved August 31, 2022.</ref> |
| | | | |
| − | Overall, the data implicating specific genes in the development of drug addiction is mixed for most genes. One reason for this may be that the case is due to a focus of current research on common variants. Many addiction studies focus on common variants with an allele frequency of greater than 5% in the general population; however, when associated with disease, these only confer a small amount of additional risk with an odds ratio of 1.1–1.3 percent. On the other hand, the rare variant hypothesis states that genes with low frequencies in the population (<1%) confer much greater additional risk in the development of the disease.<ref name="pmid23454283">{{cite journal | vauthors = Clarke TK, Crist RC, Kampman KM, Dackis CA, Pettinati HM, O'Brien CP, Oslin DW, Ferraro TN, Lohoff FW, Berrettini WH | title = Low frequency genetic variants in the μ-opioid receptor (OPRM1) affect risk for addiction to heroin and cocaine | journal = Neuroscience Letters | volume = 542 | issue = | pages = 71–75 | year = 2013 | pmid = 23454283 | doi = 10.1016/j.neulet.2013.02.018 | pmc=3640707}}</ref>
| + | =====Cannabinoid addiction===== |
| | + | [[Cannabis]] is a widely used substance, and demand for effective treatment is increasing. However, abstinence rates following behavioral therapies have been modest, and there are no effective pharmacotherapies for the treatment of cannabis addiction.<ref>Mehmet Sofuoglu, Dawn E. Sugarman, and Kathleen M. Carroll, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2909584/ Cognitive Function as an Emerging Treatment Target for Marijuana Addiction] ''Exp Clin Psychopharmacol.'' 18(2) (2010): 109–119. Retrieved August 31, 2022.</ref> |
| | | | |
| − | [[Genome-wide association study|Genome-wide association studies]] (GWAS) are used to examine genetic associations with dependence, addiction, and drug use. These studies employ an unbiased approach to finding genetic associations with specific phenotypes and give equal weight to all regions of DNA, including those with no ostensible relationship to drug metabolism or response. These studies rarely identify genes from proteins previously described via animal knockout models and candidate gene analysis. Instead, large percentages of genes involved in processes such as cell adhesion are commonly identified. This is not to say that previous findings, or the GWAS findings, are erroneous. The important effects of [[endophenotype]]s are typically not capable of being captured by these methods. Furthermore, genes identified in GWAS for drug addiction may be involved either in adjusting brain behavior prior to drug experiences, subsequent to them, or both.<ref>{{cite journal|last1=Hall|first1=F. Scott|author2=Drgonova, Jana |author3=Jain, Siddharth |author4=Uhl, George R.|title=Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong?|journal=Pharmacology & Therapeutics|date=December 2013|volume=140|issue=3|pages=267–79|doi=10.1016/j.pharmthera.2013.07.006|pmid=23872493|pmc=3797854}}</ref> | + | =====Nicotine addiction===== |
| | + | Medication assisted treatment has been widely used is in the treatment of [[nicotine]] addiction. This usually involves [[nicotine replacement therapy]], [[nicotinic receptor antagonist]]s, or [[nicotinic receptor]] [[partial agonist]]s. Drugs that act on nicotinic receptors and have been used for treating nicotine addiction include antagonists like [[bupropion]] and the partial agonist [[varenicline]].<ref>Peter A. Crooks, Michael T. Bardo, and Linda P. Dwoskin, [https://pubmed.ncbi.nlm.nih.gov/24484986/ Nicotinic Receptor Antagonists as Treatments for Nicotine Abuse] ''Adv Pharmacol'' 69 (2014):513-551. Retrieved August 31, 2022. </ref> |
| | | | |
| − | A study that highlights the significant role genetics play in addiction is the twin studies. Twins have similar and sometimes identical genetics. Analyzing these genes in relation to genetics has helped geneticists understand how much of a role genes play in addiction. Studies performed on twins found that rarely did only one twin have an addiction. In most cases where at least one twin suffered from an addiction, both did, and often to the same substance.<ref name="Crowe1991">{{cite journal|last1=Crowe|first1=J.R|title=Genetics of alcoholism|journal=Alcohol Health and Research World|pages=1–11|url=http://psycnet.apa.org/record/1993-22020-001|accessdate=13 December 2017}}</ref> Cross addiction is when already has a predisposed addiction and then starts to become addicted to something different. If one family member has a history of addiction, the chances of a relative or close family developing those same habits are much higher than one who has not been introduced to addiction at a young age.<ref>{{Cite news|url=https://www.addictionsandrecovery.org/is-addiction-a-disease.htm|title=The Genetics of Addiction – Is Addiction a Disease?|last=Life|first=Dr. Steven Melemis, I Want to Change My|work=I Want to Change My Life|access-date=17 September 2018}}</ref> In a recent study done by the National Institute on Drug Abuse, from 2002 to 2017, overdose deaths have almost tripled amongst male and females. In 2017, 72,306 overdose deaths happened in the U.S. that were reported.<ref>{{Cite news|url=https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates|title=Overdose Death Rates|last=Abuse|first=National Institute on Drug|date=9 August 2018|access-date=17 September 2018}}</ref>
| + | =====Opioid addiction===== |
| | + | [[Opioid]]s cause [[physical dependence]], and treatment typically addresses both dependence and addiction. |
| | | | |
| − | ===Environmental factors===
| + | Physical dependence is treated using replacement drugs such as [[suboxone]] or [[subutex]] (both containing the active ingredients [[buprenorphine]]) and [[methadone]].<ref>M. Connock et al., [https://pubmed.ncbi.nlm.nih.gov/17313907/ Methadone and Buprenorphine for the Management of Opioid Dependence: A Systematic Review and Economic Evaluation] ''Health Technol Assess'' 11(9) (2007):1-171. Retrieved August 31, 2022.</ref> Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a measure of control over both pain and cravings. Use of replacement drugs increases the addicted individual's ability to function normally and eliminates the negative consequences of obtaining controlled substances illicitly. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases. |
| | | | |
| − | Environmental risk factors for addiction are the experiences of an individual during their lifetime that interact with the individual's genetic composition to increase or decrease his or her vulnerability to addiction.<ref name="Cellular basis" /> A number of different environmental factors have been implicated as risk factors for addiction, including various psychosocial stressors;<ref name="Cellular basis" /> however, an individual's exposure to an addictive drug is by far the most significant environmental risk factor for addiction.<ref name="Cellular basis" /> The [[National Institute on Drug Abuse]] (NIDA) cites lack of parental supervision, the prevalence of peer substance use, drug availability, and poverty as risk factors for substance use among children and adolescents.<ref name="Abuse">{{Cite news|url=https://www.drugabuse.gov/publications/preventing-drug-abuse-among-children-adolescents/chapter-1-risk-factors-protective-factors/what-are-risk-factors|title=What are risk factors and protective factors?|last=Abuse|first=National Institute on Drug|access-date=13 December 2017}}</ref>
| + | In the United States, opiate replacement therapy is tightly regulated in [[methadone clinic]]s and under the [[DATA 2000]] legislation. In some countries, other opioid derivatives are used as substitute drugs for illegal street opiates, with different prescriptions being given depending on the needs of the individual patient. |
| | | | |
| − | Adverse childhood experiences (ACEs) are various forms of [[child maltreatment|maltreatment]] and household dysfunction experienced in childhood. The [[Adverse Childhood Experiences Study]] by the [[Centers for Disease Control and Prevention]] has shown a strong [[dose–response relationship]] between ACEs and numerous health, social, and behavioral problems throughout a person's lifespan, including those associated with [[substance abuse]].<ref name=SAMHSA_ACE/> Children's neurological development can be permanently disrupted when they are chronically exposed to stressful events such as physical, emotional, or sexual abuse, physical or emotional neglect, witnessing violence in the household, or a parent being incarcerated or suffering from a mental illness. As a result, the child's cognitive functioning or ability to cope with negative or disruptive emotions may be impaired. Over time, the child may adopt substance use as a coping mechanism, particularly during [[adolescence]].<ref name=SAMHSA_ACE>{{cite web |title=Adverse Childhood Experiences |publisher=Substance Abuse and Mental Health Services Administration |location=Rockville, Maryland, United States |website=samhsa.gov |url=http://www.samhsa.gov/capt/practicing-effective-prevention/prevention-behavioral-health/adverse-childhood-experiences |accessdate=26 September 2016}}</ref> A study of 900 court cases involving children who experienced abuse found that a vast amount of them went on to suffer from some form of addiction in their adolescence or adult life.<ref name="Enoch2011" /> This pathway towards addiction that is opened through stressful experiences during childhood can be avoided by a change in environmental factors throughout an individual's life and opportunities of professional help.<ref name="Enoch2011">{{cite journal|last1=Enoch|first1=Mary|title=The role of early life stress as a predictor for alcohol and drug dependence|journal=Psychopharmacology|pages=17–31|pmid=20596857|doi=10.1007/s00213-010-1916-6|pmc=3005022|volume=214|issue=1|year=2011}}</ref> If one has friends or peers who engage in drug use favorably, the chances of them developing an addiction increases. Family conflict and home management is also a cause for one to become engaged in alcohol or other drug use.<ref>{{Cite web |url=https://learn.genetics.utah.edu/content/addiction/environment/ |title=Environmental Risk Factors |website=learn.genetics.utah.edu |access-date=17 September 2018 }}</ref>
| + | =====Psychostimulant addiction===== |
| − | <!--[[Psychological trauma|Traumatic experiences]] have been linked to the development of [[Substance-related disorder|addictive and substance-related problems]].<ref name="pmid8711018">{{cite journal | vauthors = Stewart SH | title = Alcohol abuse in individuals exposed to trauma: a critical review | journal = Psychological Bulletin | volume = 120 | issue = 1 | pages = 83–112 | date = July 1996 | pmid = 8711018 | doi = 10.1037/0033-2909.120.1.83| url = }}</ref><ref>{{cite book | vauthors = Bailey KM, Stewart SH | chapter = Relations among trauma, PTSD, and substance misuse: The scope of the problem. | title = Trauma and Substance Abuse: Causes, Consequences, and Treatment of Comorbid Disorders | edition = 2nd | veditors = Ouimette P, Read JP | publisher = American Psychological Association | location = Washington, DC| date = 2014 | pages = 11–34 }}</ref> Interpersonal traumas, such as [[child abuse]] and [[Assault|violent assaults]], are particularly predictive of substance-related problems.<ref>{{cite journal | vauthors = Guina J, Nahhas RW, Goldberg AJ, Farnsworth S | title = PTSD Symptom Severities, Interpersonal Traumas, and Benzodiazepines Are Associated with Substance-Related Problems in Trauma Patients | journal = Journal of Clinical Medicine | volume = 5 | issue = 8 | pages = 70 | date = August 2016 | pmid = 27517964 | pmc = 4999790 | doi = 10.3390/jcm5080070 }}</ref><ref>Ford, J.D.; Elhai, J.D.; Connor, D.F.; Frueh, B.C. Poly-victimization and risk of posttraumatic, depressive, and substance use disorders and involvement in delinquency in a national sample of adolescents. J. Adolesc. Health 2010, 46, 545–52.</ref><ref>{{cite journal = Sullivan TP, Cavanaugh CE, Buckner JD, Edmondson D | title = Intimate partner violence (IPV) and drug and alcohol use problems among community women: The roles of physical, sexual, and psychological IPV and PTSD. | journal = J. Trauma Stress | date = 2009 | volume = 22 | pages = 575–84 }}</ref>
| + | There is no effective [[pharmacotherapy]] for any form of psychostimulant addiction. Many drugs have been tested, but none have shown conclusive efficacy with tolerable side effects in humans.<ref name=Taylor/> Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved, and psychotherapy remains the mainstay of treatment. |
| − | —>
| |
| | | | |
| − | ==== Age ==== | + | ===Risk factors=== |
| | + | There are many known risk factors associated with an increased chance of developing a substance use disorder (SUD). For example, children born to parents with SUDs have roughly a two-fold increased risk in developing an addiction compared to children born to parents without any SUDs. Other common risk factors are being male, being under 25, having other mental health problems, and lack of familial support and supervision.<ref> Fred F. Ferri, ''Ferri's Clinical Advisor 2020'' (Elsevier, 2019, ISBN 978-0323672542).</ref> Psychological risk factors include high [[impulsivity]], [[sensation seeking]], [[neuroticism]], and [[openness to experience]] in combination with low [[conscientiousness]].<ref>Elaine Fehrman, Vincent Egan, Alexander N. Gorban, Jeremy Levesley, Evgeny M. Mirkes, and Awaz K. Muhammad, ''Personality Traits and Drug Consumption: A Story Told by Data'' (Springer, 2019, ISBN 978-3030104412).</ref> |
| | | | |
| − | Adolescence represents a period of unique vulnerability for developing an addiction.<ref>{{cite journal | vauthors = Spear LP | title = The adolescent brain and age-related behavioral manifestations | journal = Neuroscience and Biobehavioral Reviews | volume = 24 | issue = 4 | pages = 417–63 | date = June 2000 | pmid = 10817843 | doi = 10.1016/s0149-7634(00)00014-2 | citeseerx = 10.1.1.461.3295 }}</ref> In adolescence, the incentive-rewards systems in the brain mature well before the cognitive control center. This consequentially grants the incentive-rewards systems a disproportionate amount of power in the behavioral decision-making process. Therefore, adolescents are increasingly likely to act on their impulses and engage in risky, potentially addicting behavior before considering the consequences.<ref>{{cite journal | vauthors = Hammond CJ, Mayes LC, Potenza MN | title = Neurobiology of adolescent substance use and addictive behaviors: treatment implications | journal = Adolescent Medicine | volume = 25 | issue = 1 | pages = 15–32 | date = April 2014 | pmid = 25022184 | pmc = 4446977 }}</ref> Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.<ref>{{cite journal | vauthors = Catalano RF, Hawkins JD, Wells EA, Miller J, Brewer D | title = Evaluation of the effectiveness of adolescent drug abuse treatment, assessment of risks for relapse, and promising approaches for relapse prevention | journal = The International Journal of the Addictions | volume = 25 | issue = 9A–10A | pages = 1085–140 | pmid = 2131328 | year=1990| doi = 10.3109/10826089109081039 }}</ref><ref>{{cite journal | vauthors = Perepletchikova F, Krystal JH, Kaufman J | title = Practitioner review: adolescent alcohol use disorders: assessment and treatment issues | journal = Journal of Child Psychology and Psychiatry, and Allied Disciplines | volume = 49 | issue = 11 | pages = 1131–54 | date = November 2008 | pmid = 19017028 | pmc = 4113213 | doi = 10.1111/j.1469-7610.2008.01934.x }}</ref>
| + | There are a number of genetic and environmental risk factors for developing an addiction, that vary across the population. Even in individuals with a relatively low genetic risk, exposure to sufficiently high doses of an addictive drug for a long period of time can result in an addiction.<ref name="Cellular basis" /> |
| | | | |
| − | Statistics have shown that those who start to drink alcohol at a younger age are more likely to become dependent later on. About 33% of the population tasted their first alcohol between the ages of 15 and 17, while 18% experienced it prior to this. As for alcohol abuse or dependence, the numbers start off high with those who first drank before they were 12 and then drop off after that. For example, 16% of alcoholics began drinking prior to turning 12 years old, while only 9% first touched alcohol between 15 and 17. This percentage is even lower, at 2.6%, for those who first started the habit after they were 21.<ref>{{cite web|url=http://alcoholrehab.com/drug-addiction/age-and-substance-abuse/|title=Age and Substance Abuse – Alcohol Rehab}}</ref>
| + | ==== Genetic factors ==== |
| | + | It has long been established that genetic factors along with environmental (such as psychosocial) factors are significant contributors to addiction vulnerability.<ref name="Cellular basis" /> [[Epidemiological method|Epidemiological]] studies estimate that genetic factors account for 40–60 percent of the risk factors for [[alcoholism]].<ref>R.D. Mayfield, R.A. Harris, and M.A. Schuckit, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2442454/ Genetic factors influencing alcohol dependence] ''Br J Pharmacol'' 154(2) (2008): 275–287. Retrieved August 31, 2022.</ref> Similar rates of heritability for other types of drug addiction have been indicated by other studies.<ref name=Melemis>Steven M. Melemis, [https://www.addictionsandrecovery.org/is-addiction-a-disease.htm The Genetics of Drug and Alcohol Addiction] ''Addictions and Recovery''. Retrieved August 31, 2022.</ref> |
| | | | |
| − | Most individuals are exposed to and use addictive drugs for the first time during their teenage years.<ref name="Drug use trends" /> In the United States, there were just over 2.8 million new users of illicit drugs in 2013 (~7,800 new users per day);<ref name="Drug use trends" /> among them, 54.1% were under 18 years of age.<ref name="Drug use trends">{{cite web|title=Nationwide Trends|url=http://www.drugabuse.gov/publications/drugfacts/nationwide-trends|publisher=National Institute on Drug Abuse|accessdate=15 December 2017|date=June 2015}}</ref> In 2011, there were approximately 20.6 million people in the United States over the age of 12 with an addiction.<ref name=":0">{{Cite news|url=https://www.addictioncenter.com/addiction/addiction-statistics/|title=Addiction Statistics – Facts on Drug and Alcohol Addiction|work=AddictionCenter|access-date=17 September 2018}}</ref> Over 90% of those with an addiction began drinking, smoking or using illicit drugs before the age of 18.<ref name=":0" />
| + | Twin studies highlight the significant role genetics play in addiction. Rarely does only one twin have an addiction: In most cases where at least one twin suffered from an addiction, both did, and often to the same substance. Family studies reveal that if one family member has a history of addiction, the chances of a relative or close family developing an addiction to the same substance or a different addiction are much higher than one who has not been introduced to addiction at a young age. Such "cross addiction" occurs because all addictions work in the same part of the brain.<ref name=Melemis/> |
| | | | |
| − | ==== Comorbid disorders ==== | + | ====Environmental factors==== |
| − | Individuals with [[comorbid]] (i.e., co-occurring) [[mental health]] disorders such as depression, anxiety, attention-deficit/hyperactivity disorder (ADHD) or post-traumatic stress disorder are more likely to develop substance use disorders.<ref>{{Cite web|url=https://www.samhsa.gov/capt/practicing-effective-prevention/prevention-behavioral-health/risk-protective-factors|title=Risk and Protective Factors|last=SAMHSA|date=|publisher=Substance Abuse and Mental Health Administration|access-date=19 December 2016}}</ref><ref>{{Cite web|url=http://www.recoveryanswers.org/pressrelease/infographic-risk-factors-addiction/|title=Infographic – Risk Factors of Addiction {{!}} Recovery Research Institute|website=www.recoveryanswers.org|access-date=19 December 2016|archive-url=https://web.archive.org/web/20161217014124/http://www.recoveryanswers.org/pressrelease/infographic-risk-factors-addiction/|archive-date=17 December 2016|url-status=dead}}</ref><ref>{{Cite web|url=http://www.mayoclinic.org/diseases-conditions/drug-addiction/basics/risk-factors/con-20020970|title=Drug addiction Risk factors – Mayo Clinic|website=www.mayoclinic.org|access-date=19 December 2016}}</ref> The {{Abbrlink|NIDA|National Institute on Drug Abuse}} cites early aggressive behavior as a risk factor for substance use.<ref name="Abuse"/> A study by the [[National Bureau of Economic Research]] found that there is a "definite connection between mental illness and the use of addictive substances" and a majority of mental health patients participate in the use of these substances: 38% alcohol, 44% cocaine, and 40% cigarettes.<ref>{{Cite news|url=https://www.dualdiagnosis.org/mental-health-and-addiction/the-connection/|title=The Connection Between Mental Illness and Substance Abuse {{!}} Dual Diagnosis|work=Dual Diagnosis|access-date=17 September 2018}}</ref>
| + | A number of different environmental factors have been implicated as risk factors for addiction, including various psychosocial stressors. However, an individual's exposure to an addictive drug is by far the most significant environmental risk factor for addiction.<ref name="Cellular basis" /> The [[National Institute on Drug Abuse]] (NIDA) cites lack of parental supervision, the prevalence of peer substance use, drug availability, and poverty as risk factors for substance use among children and adolescents.<ref> What are risk factors and protective factors? ''National Institute on Drug Abuse'', October 2011.</ref> |
| | | | |
| − | ===Epigenetic factors=== | + | ===== Age ===== |
| | + | The earlier someone starts to use drugs, the higher the chance that they will grow to abuse or become dependent on them later on in life. Statistics have shown that those who start to drink alcohol at a younger age, especially prior to 12 years of age, are more likely to become dependent later on.<ref>[https://alcoholrehab.com/drug-addiction/effects/age-and-substance-abuse// Age and Substance Abuse] ''Alcohol Rehab''. Retrieved August 31, 2022.</ref> |
| | | | |
| − | ==== Transgenerational epigenetic inheritance ====
| + | [[Adolescence]] represents a period of unique vulnerability for developing an addiction. In adolescence, the incentive-rewards systems in the brain mature well before the cognitive control center. This consequentially grants the incentive-rewards systems a disproportionate amount of power in the behavioral decision-making process. Therefore, adolescents are increasingly likely to act on their impulses and engage in risky, potentially addicting behavior before considering the consequences.<ref>Christopher J. Hammond, Linda C. Mayes, and Marc N. Potenza, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4446977/ Neurobiology of Adolescent Substance Use and Addictive Behaviors: Prevention and Treatment Implications] ''Adolesc Med State Art Rev'' 25(1) (2014): 15–32. Retrieved August 31, 2022.</ref> Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse. |
| − | {{See also|Transgenerational epigenetic inheritance}}
| |
| − | [[Epigenetic]] genes and their products (e.g., proteins) are the key components through which environmental influences can affect the genes of an individual;<ref name="Transgenerational epigenetic inheritance in addiction" /> they also serve as the mechanism responsible for [[transgenerational epigenetic inheritance]], a phenomenon in which environmental influences on the genes of a parent can affect the associated traits and [[behavioral phenotype]]s of their offspring (e.g., behavioral responses to environmental stimuli).<ref name="Transgenerational epigenetic inheritance in addiction" /> In addiction, epigenetic mechanisms play a central role in the [[pathophysiology]] of the disease;<ref name="Cellular basis" /> it has been noted that some of the alterations to the [[epigenome]] which arise through chronic exposure to addictive stimuli during an addiction can be transmitted across generations, in turn affecting the behavior of one's children (e.g., the child's behavioral responses to addictive drugs and [[natural reward]]s).<ref name="Transgenerational epigenetic inheritance in addiction">{{cite journal | vauthors = Vassoler FM, Sadri-Vakili G | title = Mechanisms of transgenerational inheritance of addictive-like behaviors | journal = Neuroscience | volume = 264 | issue = | pages = 198–206 | year = 2014 | pmid = 23920159 | pmc = 3872494 | doi = 10.1016/j.neuroscience.2013.07.064 | quote = However, the components that are responsible for the heritability of characteristics that make an individual more susceptible to drug addiction in humans remain largely unknown given that patterns of inheritance cannot be explained by simple genetic mechanisms (Cloninger et al., 1981; Schuckit et al., 1972). The environment also plays a large role in the development of addiction as evidenced by great societal variability in drug use patterns between countries and across time (UNODC, 2012). Therefore, both genetics and the environment contribute to an individual's vulnerability to become addicted following an initial exposure to drugs of abuse. ...<br />The evidence presented here demonstrates that rapid environmental adaptation occurs following exposure to a number of stimuli. Epigenetic mechanisms represent the key components by which the environment can influence genetics, and they provide the missing link between genetic heritability and environmental influences on the behavioral and physiological phenotypes of the offspring.}}</ref><ref name="pmid26572641">{{cite journal | vauthors = Yuan TF, Li A, Sun X, Ouyang H, Campos C, Rocha NB, Arias-Carrión O, Machado S, Hou G, So KF | title = Transgenerational Inheritance of Paternal Neurobehavioral Phenotypes: Stress, Addiction, Ageing and Metabolism | journal = Mol. Neurobiol. | volume = 53| issue = 9| pages = 6367–76| year = 2015 | pmid = 26572641 | doi = 10.1007/s12035-015-9526-2 | url = | hdl = 10400.22/7331 | hdl-access = free }}</ref> | |
| | | | |
| − | The general classes of epigenetic alterations that have been implicated in transgenerational epigenetic inheritance include [[DNA methylation]], [[histone modification]]s, and [[Downregulation and upregulation|downregulation or upregulation]] of [[microRNA]]s.<ref name="Transgenerational epigenetic inheritance in addiction" /> With respect to addiction, more research is needed to determine the specific [[Heredity|heritable]] epigenetic alterations that arise from various forms of addiction in humans and the corresponding behavioral phenotypes from these epigenetic alterations that occur in human offspring.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" /> Based upon preclinical evidence from [[animal research]], certain addiction-induced epigenetic alterations in rats can be transmitted from parent to offspring and produce behavioral phenotypes that decrease the offspring's risk of developing an addiction.{{#tag:ref|According to a review of experimental animal models that examined the transgenerational epigenetic inheritance of [[epigenetic mark]]s that occur in addiction, alterations in [[histone acetylation]] – specifically, di-acetylation of [[lysine]] [[residue (amino acid)|residues]] 9 and 14 on [[histone 3]] (i.e., [[H3K9ac2]] and [[H3K14ac2]]) in association with [[BDNF]] [[gene promoter]]s – have been shown to occur within the [[medial prefrontal cortex]] (mPFC), [[testes]], and [[sperm]] of cocaine-addicted male rats.<ref name="Transgenerational epigenetic inheritance in addiction" /> These epigenetic alterations in the rat mPFC result in increased BDNF [[gene expression]] within the mPFC, which in turn blunts the [[reward system|rewarding properties]] of cocaine and reduces cocaine [[self-administration]].<ref name="Transgenerational epigenetic inheritance in addiction" /> The male but not female offspring of these cocaine-exposed rats inherited both epigenetic marks (i.e., di-acetylation of lysine residues 9 and 14 on histone 3) within mPFC neurons, the corresponding increase in BDNF expression within mPFC neurons, and the behavioral phenotype associated with these effects (i.e., a reduction in cocaine reward, resulting in reduced cocaine-seeking by these male offspring).<ref name="Transgenerational epigenetic inheritance in addiction" /> Consequently, the transmission of these two cocaine-induced epigenetic alterations (i.e., H3K9ac2 and H3K14ac2) in rats from male fathers to male offspring served to reduce the offspring's risk of developing an addiction to cocaine.<ref name="Transgenerational epigenetic inheritance in addiction" /> {{As of|2018|post=,}} neither the heritability of these epigenetic marks in humans nor the behavioral effects of the marks within human mPFC neurons has been established.<ref name="Transgenerational epigenetic inheritance in addiction" />|group="note"}}<ref name="Transgenerational epigenetic inheritance in addiction" /> More generally, the heritable behavioral phenotypes that are derived from addiction-induced epigenetic alterations and transmitted from parent to offspring may serve to either increase or decrease the offspring's risk of developing an addiction.<ref name="Transgenerational epigenetic inheritance in addiction" /><ref name="pmid26572641" />
| + | Most individuals are exposed to and use addictive drugs for the first time during their teenage years. In the United States, for example, over 90 percent of those with an addiction began drinking, smoking, or using illicit drugs before the age of 18.<ref>[https://www.addictioncenter.com/addiction/addiction-statistics/ Statistics on Addiction in America] ''Addiction Center''. Retrieved August 31, 2022.</ref> |
| | | | |
| − | ==Mechanisms== | + | ===== Comorbid disorders ===== |
| − | Addiction is a disorder of the brain's [[reward system]] which arises through [[transcriptional]] and [[epigenetic]] mechanisms and develops over time from chronically high levels of exposure to an addictive stimulus (e.g., eating food, the use of cocaine, engagement in sexual activity, participation in high-thrill cultural activities such as gambling, etc.).<ref name="Cellular basis" /><ref name="What the ΔFosB?" /><ref name="Natural and drug addictions" /> [[FOSB#DeltaFosB|DeltaFosB]] (ΔFosB), a gene [[transcription factor]], is a critical component and common factor in the development of virtually all forms of behavioral and drug addictions.<ref name="What the ΔFosB?" /><ref name="Natural and drug addictions" /><ref name="G9a reverses ΔFosB plasticity" /><ref name="Nestler" /> Two decades of research into ΔFosB's role in addiction have demonstrated that addiction arises, and the associated compulsive behavior intensifies or attenuates, along with the [[overexpression]] of ΔFosB in the [[D1-type]] [[medium spiny neuron]]s of the [[nucleus accumbens]].<ref name="Cellular basis" /><ref name="What the ΔFosB?" /><ref name="Natural and drug addictions" /><ref name="G9a reverses ΔFosB plasticity" /> Due to the causal relationship between ΔFosB expression and addictions, it is used [[preclinical research|preclinically]] as an addiction [[biomarker (medicine)|biomarker]].<ref name="Cellular basis" /><ref name="What the ΔFosB?">{{cite journal | author = Ruffle JK | title = Molecular neurobiology of addiction: what's all the (Δ)FosB about? | journal = Am. J. Drug Alcohol Abuse | volume = 40 | issue = 6 | pages = 428–37 | date = November 2014 | pmid = 25083822 | doi = 10.3109/00952990.2014.933840 | quote = <br />The strong correlation between chronic drug exposure and ΔFosB provides novel opportunities for targeted therapies in addiction (118), and suggests methods to analyze their efficacy (119). Over the past two decades, research has progressed from identifying ΔFosB induction to investigating its subsequent action (38). It is likely that ΔFosB research will now progress into a new era – the use of ΔFosB as a biomarker. ...<br />Conclusions<br />ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a ''molecular switch'' (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). Some of these proposed interventions have limitations (125) or are in their infancy (75). However, it is hoped that some of these preliminary findings may lead to innovative treatments, which are much needed in addiction.}}</ref><ref name="G9a reverses ΔFosB plasticity">{{cite journal | vauthors = Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T | title = Epigenetic regulation in drug addiction | journal = Ann. Agric. Environ. Med. | volume = 19 | issue = 3 | pages = 491–96 | year = 2012 | pmid = 23020045 | doi = | quote = For these reasons, ΔFosB is considered a primary and causative transcription factor in creating new neural connections in the reward centre, prefrontal cortex, and other regions of the limbic system. This is reflected in the increased, stable and long-lasting level of sensitivity to cocaine and other drugs, and tendency to relapse even after long periods of abstinence. These newly constructed networks function very efficiently via new pathways as soon as drugs of abuse are further taken ... In this way, the induction of CDK5 gene expression occurs together with suppression of the G9A gene coding for dimethyltransferase acting on the histone H3. A feedback mechanism can be observed in the regulation of these 2 crucial factors that determine the adaptive epigenetic response to cocaine. This depends on ΔFosB inhibiting G9a gene expression, i.e. H3K9me2 synthesis which in turn inhibits transcription factors for ΔFosB. For this reason, the observed hyper-expression of G9a, which ensures high levels of the dimethylated form of histone H3, eliminates the neuronal structural and plasticity effects caused by cocaine by means of this feedback which blocks ΔFosB transcription}}</ref> ΔFosB expression in these neurons directly and positively regulates drug [[self-administration]] and [[#Sensitization|reward sensitization]] through [[positive reinforcement]], while decreasing sensitivity to [[wikt:aversion|aversion]].{{#tag:ref|A decrease in aversion sensitivity, in simpler terms, means that an individual's behavior is less likely to be influenced by undesirable outcomes.|group="note"|name="ΔFosB behaviors"}}<ref name="Cellular basis" /><ref name="What the ΔFosB?" />
| + | Individuals with [[comorbid]] (co-occurring) [[mental health]] disorders such as [[Clinical depression|depression]], [[Anxiety disorder|anxiety]], attention-deficit/hyperactivity disorder ([[ADHD]]), or post-traumatic stress disorder ([[PTSD]]) are more likely to develop substance use disorders.<ref>[https://www.mayoclinic.org/diseases-conditions/drug-addiction/symptoms-causes/syc-20365112 Drug addiction (substance use disorder): Risk factors] ''Mayo Clinic''. Retrieved August 31, 2022.</ref> The [[National Bureau of Economic Research]] reports a "definite connection between mental illness and the use of addictive substances," and "When other factors are held constant, mental illness does increase use of addictive goods — relative to use by the overall population — by 20 percent for alcohol, 27 percent for cocaine, and 86 percent for cigarettes."<ref>Marie Bussing-Birks, [https://www.nber.org/digest/apr02/mental-illness-and-substance-abuse Mental Illness and Substance Abuse] ''National Bureau of Economic Research''. Retrieved August 31, 2022.</ref> |
| | | | |
| | + | ====Epigenetic factors==== |
| | + | Transgenerational [[epigenetic]] [[inheritance]] is the transmission of epigenetic markers from one generation to the next (parent–child transmission), affecting the traits and [[behavioral phenotype]]s of their offspring (for example, behavioral responses to environmental stimuli) without alteration of the primary structure of [[DNA]] (the sequence of [[nucleotide]]s). In addiction, epigenetic mechanisms play a central role in the [[pathophysiology]] of the disease.<ref name="Cellular basis" /> Some of the alterations to the [[epigenome]] which arise through chronic exposure to addictive stimuli during an addiction can be transmitted across generations, in turn affecting the behavior of one's children (such as the child's behavioral responses to addictive drugs and [[natural reward]]s).<ref name="Transgenerational epigenetic inheritance in addiction">F.M. Vassoler and G. Sadri-Vakili, [https://pubmed.ncbi.nlm.nih.gov/23920159/ Mechanisms of transgenerational inheritance of addictive-like behaviors] ''Neuroscience'' 264 (2014): 198–206. Retrieved August 31, 2022.</ref> However, the components that are responsible for the heritability of characteristics that make an individual more susceptible to drug addiction in humans remain largely unknown. |
| | | | |
| − | [[Cognitive control]] and [[stimulus control]], which is associated with [[operant conditioning|operant]] and [[classical conditioning]], represent opposite processes (i.e., internal vs external or environmental, respectively) that compete over the control of an individual's elicited behaviors.<ref name="Cognitive - stimulus">{{cite journal | vauthors = Washburn DA | title = The Stroop effect at 80: The competition between stimulus control and cognitive control | journal = J Exp Anal Behav | volume = 105 | issue = 1 | pages = 3–13 | year = 2016 | pmid = 26781048 | doi = 10.1002/jeab.194 | quote = Today, arguably more than at any time in history, the constructs of attention, executive functioning, and cognitive control seem to be pervasive and preeminent in research and theory. Even within the cognitive framework, however, there has long been an understanding that behavior is multiply determined, and that many responses are relatively automatic, unattended, contention-scheduled, and habitual. Indeed, the cognitive flexibility, response inhibition, and self-regulation that appear to be hallmarks of cognitive control are noteworthy only in contrast to responses that are relatively rigid, associative, and involuntary. }}</ref> Cognitive control, and particularly [[inhibitory control|inhibitory control over behavior]], is impaired in both addiction and [[attention deficit hyperactivity disorder]].<ref name="Executive functions">{{cite journal | author = Diamond A | title = Executive functions | journal = Annu Rev Psychol | volume = 64 | issue = | pages = 135–68 | year = 2013 | pmid = 23020641 | pmc = 4084861 | doi = 10.1146/annurev-psych-113011-143750 | quote = Core EFs are inhibition [response inhibition (self-control – resisting temptations and resisting acting impulsively) and interference control (selective attention and cognitive inhibition)], working memory, and cognitive flexibility (including creatively thinking "outside the box," seeing anything from different perspectives, and quickly and flexibly adapting to changed circumstances). ... EFs and prefrontal cortex are the first to suffer, and suffer disproportionately, if something is not right in your life. They suffer first, and most, if you are stressed (Arnsten 1998, Liston et al. 2009, Oaten & Cheng 2005), sad (Hirt et al. 2008, von Hecker & Meiser 2005), lonely (Baumeister et al. 2002, Cacioppo & Patrick 2008, Campbell et al. 2006, Tun et al. 2012), sleep deprived (Barnes et al. 2012, Huang et al. 2007), or not physically fit (Best 2010, Chaddock et al. 2011, Hillman et al. 2008). Any of these can cause you to appear to have a disorder of EFs, such as ADHD, when you do not. You can see the deleterious effects of stress, sadness, loneliness, and lack of physical health or fitness at the physiological and neuroanatomical level in prefrontal cortex and at the behavioral level in worse EFs (poorer reasoning and problem solving, forgetting things, and impaired ability to exercise discipline and self-control). ...<br />EFs can be improved (Diamond & Lee 2011, Klingberg 2010). ... At any age across the life cycle EFs can be improved, including in the elderly and in infants. There has been much work with excellent results on improving EFs in the elderly by improving physical fitness (Erickson & Kramer 2009, Voss et al. 2011) ... Inhibitory control (one of the core EFs) involves being able to control one's attention, behavior, thoughts, and/or emotions to override a strong internal predisposition or external lure, and instead do what's more appropriate or needed. Without inhibitory control we would be at the mercy of impulses, old habits of thought or action (conditioned responses), and/or stimuli in the environment that pull us this way or that. Thus, inhibitory control makes it possible for us to change and for us to choose how we react and how we behave rather than being unthinking creatures of habit. It doesn’t make it easy. Indeed, we usually are creatures of habit and our behavior is under the control of environmental stimuli far more than we usually realize, but having the ability to exercise inhibitory control creates the possibility of change and choice. ... The subthalamic nucleus appears to play a critical role in preventing such impulsive or premature responding (Frank 2006).}}</ref><ref name="NHM-Cognitive Control">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 313–21 | edition = 2nd | chapter = Chapter 13: Higher Cognitive Function and Behavioral Control | quote ={{bull}} Executive function, the cognitive control of behavior, depends on the prefrontal cortex, which is highly developed in higher primates and especially humans.<br />{{bull}} Working memory is a short-term, capacity-limited cognitive buffer that stores information and permits its manipulation to guide decision-making and behavior. ...<br />These diverse inputs and back projections to both cortical and subcortical structures put the prefrontal cortex in a position to exert what is often called "top-down" control or cognitive control of behavior. ... The prefrontal cortex receives inputs not only from other cortical regions, including association cortex, but also, via the thalamus, inputs from subcortical structures subserving emotion and motivation, such as the amygdala (Chapter 14) and ventral striatum (or nucleus accumbens; Chapter 15). ...<br />In conditions in which prepotent responses tend to dominate behavior, such as in drug addiction, where drug cues can elicit drug seeking (Chapter 15), or in attention deficit hyperactivity disorder (ADHD; described below), significant negative consequences can result. ... ADHD can be conceptualized as a disorder of executive function; specifically, ADHD is characterized by reduced ability to exert and maintain cognitive control of behavior. Compared with healthy individuals, those with ADHD have diminished ability to suppress inappropriate prepotent responses to stimuli (impaired response inhibition) and diminished ability to inhibit responses to irrelevant stimuli (impaired interference suppression). ... <!--Inhibitory control brain structures—>Functional neuroimaging in humans demonstrates activation of the prefrontal cortex and caudate nucleus (part of the striatum) in tasks that demand inhibitory control of behavior. Subjects with ADHD exhibit less activation of the medial prefrontal cortex than healthy controls even when they succeed in such tasks and utilize different circuits. ... Early results with structural MRI show thinning of the cerebral cortex in ADHD subjects compared with age-matched controls in prefrontal cortex and posterior parietal cortex, areas involved in working memory and attention.}}</ref> Stimulus-driven behavioral responses (i.e., stimulus control) that are associated with a particular [[rewarding stimulus]] tend to dominate one's behavior in an addiction.<ref name="NHM-Cognitive Control" />
| + | ==Behavioral addictions== |
| − | {{Transcription factor glossary|width=610px}}
| + | '''Behavioral addiction''' is a form of addiction that involves a [[compulsive behavior|compulsion]] to engage in an inherently rewarding non-[[Chemical substance|substance]]-related behavior – sometimes called a "natural reward"<ref name=Robison/><ref name="Natural and drug addictions">Christopher M. Olsen, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3139704/ Natural Rewards, Neuroplasticity, and Non-Drug Addictions] ''Neuropharmacology'' 61(7) (2011): 1109–1122. Retrieved August 31, 2022.</ref> – despite adverse consequences to the person's physical, mental, social, or financial well-being.<ref> Dan J. Stein, Eric Hollander, and Barbara O. Rothbaum (eds.), ''Textbook of Anxiety Disorders'' (American Psychiatric Publishing, Inc., 2009, ISBN 1585622540).</ref><ref name=Nestleretal/> |
| − | {{Psychostimulant addiction|Colorcode=yes|align=right}}
| |
| − | Chronic addictive drug use causes alterations in [[gene expression]] in the [[mesocorticolimbic projection]].<ref name="Nestler" /><ref name="Nestler, Hyman, and Malenka 2">{{cite journal |vauthors=Hyman SE, Malenka RC, Nestler EJ |title=Neural mechanisms of addiction: the role of reward-related learning and memory |journal=Annu. Rev. Neurosci. |volume=29 |issue= |pages=565–98 |year=2006 |pmid=16776597 |doi=10.1146/annurev.neuro.29.051605.113009 |url=}}</ref><ref name="Addiction genetics">{{cite journal |vauthors=Steiner H, Van Waes V |title=Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants |journal=Prog. Neurobiol. |volume=100 |issue= |pages=60–80 |date=January 2013 |pmid=23085425 |pmc=3525776 |doi=10.1016/j.pneurobio.2012.10.001 |url=}}</ref> The most important [[transcription factor]]s that produce these alterations are [[ΔFosB]], [[cyclic adenosine monophosphate|cAMP]] response element binding protein ([[cAMP response element binding protein|CREB]]), and nuclear factor kappa B ([[nuclear factor kappa B|NF-κB]]).<ref name="Nestler" /> ΔFosB is the most significant biomolecular mechanism in addiction because the [[overexpression]] of ΔFosB in the [[D1-type]] [[medium spiny neuron]]s in the [[nucleus accumbens]] is [[necessary and sufficient]] for many of the neural adaptations and behavioral effects (e.g., expression-dependent increases in drug [[self-administration]] and [[#Sensitization|reward sensitization]]) seen in drug addiction.<ref name="Nestler" /> ΔFosB expression in [[nucleus accumbens]] [[D1-type]] [[medium spiny neuron]]s directly and positively regulates drug [[self-administration]] and [[#Sensitization|reward sensitization]] through [[positive reinforcement]] while decreasing sensitivity to [[wikt:aversion|aversion]].<ref name="ΔFosB behaviors" group="note" /><ref name="Cellular basis" /><ref name="What the ΔFosB?" /> ΔFosB has been implicated in mediating addictions to many different drugs and drug classes, including [[alcoholism|alcohol]], [[amphetamine]] and other [[substituted amphetamines]], [[cannabinoid]]s, [[cocaine]], [[methylphenidate]], [[nicotine]], [[opiates]], [[phenylcyclidine]], and [[propofol]], among others.<ref name="What the ΔFosB?" /><!--Preceding review covers ΔFosB in propofol addiction—><ref name="Nestler" /><ref name="Nestler, Hyman, and Malenka 2" /><ref name="Alcoholism ΔFosB">{{cite web | title=Alcoholism – Homo sapiens (human) | url=http://www.genome.jp/kegg-bin/show_pathway?hsa05034+2354 | work=KEGG Pathway | accessdate=10 April 2014 | author=Kanehisa Laboratories | date=2 August 2013}}</ref><ref name="MPH ΔFosB">{{cite journal | vauthors = Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P | title = Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens | journal = Proc. Natl. Acad. Sci. USA | volume = 106 | issue = 8 | pages = 2915–20 | date = February 2009 | pmid = 19202072 | pmc = 2650365 | doi = 10.1073/pnas.0813179106 | quote = <!--Despite decades of clinical use of methylphenidate for ADHD, concerns have been raised that long-term treatment of children with this medication may result in subsequent drug abuse and addiction. ... Thus, although oral administration of clinical doses of methylphenidate is not associated with euphoria or with abuse problems, nontherapeutic use of high doses or i.v. administration may lead to addiction (39, 40).—>}}</ref> [[ΔJunD]], a transcription factor, and [[EHMT2|G9a]], a [[histone methyltransferase]], both oppose the function of ΔFosB and inhibit increases in its expression.<ref name="Cellular basis" /><ref name="Nestler" /><ref name="Nestler 2014 epigenetics" /> Increases in nucleus accumbens ΔJunD expression (via [[viral vector]]-mediated gene transfer) or G9a expression (via pharmacological means) reduces, or with a large increase can even block, many of the neural and behavioral alterations that result from chronic high-dose use of addictive drugs (i.e., the alterations mediated by ΔFosB).<ref name="G9a reverses ΔFosB plasticity" /><ref name="Nestler" />
| |
| | | | |
| − | ΔFosB also plays an important role in regulating behavioral responses to [[natural reward]]s, such as palatable food, sex, and exercise.<ref name="Nestler" /><ref name="ΔFosB reward">{{cite journal |vauthors=Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M | title = Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms | journal = Journal of Psychoactive Drugs | volume = 44 | issue = 1 | pages = 38–55 | year = 2012 | pmid = 22641964 | pmc = 4040958 | doi = 10.1080/02791072.2012.662112| quote = It has been found that deltaFosB gene in the NAc is critical for reinforcing effects of sexual reward. Pitchers and colleagues (2010) reported that sexual experience was shown to cause DeltaFosB accumulation in several limbic brain regions including the NAc, medial pre-frontal cortex, VTA, caudate, and putamen, but not the medial preoptic nucleus. Next, the induction of c-Fos, a downstream (repressed) target of DeltaFosB, was measured in sexually experienced and naive animals. The number of mating-induced c-Fos-IR cells was significantly decreased in sexually experienced animals compared to sexually naive controls. Finally, DeltaFosB levels and its activity in the NAc were manipulated using viral-mediated gene transfer to study its potential role in mediating sexual experience and experience-induced facilitation of sexual performance. Animals with DeltaFosB overexpression displayed enhanced facilitation of sexual performance with sexual experience relative to controls. In contrast, the expression of DeltaJunD, a dominant-negative binding partner of DeltaFosB, attenuated sexual experience-induced facilitation of sexual performance, and stunted long-term maintenance of facilitation compared to DeltaFosB overexpressing group. Together, these findings support a critical role for DeltaFosB expression in the NAc in the reinforcing effects of sexual behavior and sexual experience-induced facilitation of sexual performance. ... both drug addiction and sexual addiction represent pathological forms of neuroplasticity along with the emergence of aberrant behaviors involving a cascade of neurochemical changes mainly in the brain's rewarding circuitry. }}</ref> Natural rewards, like drugs of abuse, [[inducible gene|induce gene expression]] of ΔFosB in the nucleus accumbens, and chronic acquisition of these rewards can result in a similar pathological addictive state through ΔFosB overexpression.<ref name="Natural and drug addictions">{{cite journal | author = Olsen CM | title = Natural rewards, neuroplasticity, and non-drug addictions | journal = Neuropharmacology | volume = 61 | issue = 7 | pages = 1109–22 |date=December 2011 | pmid = 21459101 | pmc = 3139704 | doi = 10.1016/j.neuropharm.2011.03.010 | quote = Functional neuroimaging studies in humans have shown that gambling (Breiter et al, 2001), shopping (Knutson et al, 2007), orgasm (Komisaruk et al, 2004), playing video games (Koepp et al, 1998; Hoeft et al, 2008) and the sight of appetizing food (Wang et al, 2004a) activate many of the same brain regions (i.e., the mesocorticolimbic system and extended amygdala) as drugs of abuse (Volkow et al, 2004). ... Cross-sensitization is also bidirectional, as a history of amphetamine administration facilitates sexual behavior and enhances the associated increase in NAc DA ... As described for food reward, sexual experience can also lead to activation of plasticity-related signaling cascades. The transcription factor delta FosB is increased in the NAc, PFC, dorsal striatum, and VTA following repeated sexual behavior (Wallace et al., 2008; Pitchers et al., 2010b). This natural increase in delta FosB or viral overexpression of delta FosB within the NAc modulates sexual performance, and NAc blockade of delta FosB attenuates this behavior (Hedges et al, 2009; Pitchers et al., 2010b). Further, viral overexpression of delta FosB enhances the conditioned place preference for an environment paired with sexual experience (Hedges et al., 2009). ... In some people, there is a transition from "normal" to compulsive engagement in natural rewards (such as food or sex), a condition that some have termed behavioral or non-drug addictions (Holden, 2001; Grant et al., 2006a). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al, 2006; Aiken, 2007; Lader, 2008)."}}<br /><!--The following link is outside the template to make it hyperlinked while appearing to be part of the quote.—>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3139704/table/T1/ Table 1: Summary of plasticity observed following exposure to drug or natural reinforcers]"</ref><ref name="Nestler" /><ref name="ΔFosB reward" /> Consequently, ΔFosB is the key transcription factor involved in addictions to natural rewards (i.e., behavioral addictions) as well;<ref name="Nestler">{{cite journal |vauthors=Robison AJ, Nestler EJ | title = Transcriptional and epigenetic mechanisms of addiction | journal = Nat. Rev. Neurosci. | volume = 12 | issue = 11 | pages = 623–37 |date=November 2011 | pmid = 21989194 | pmc = 3272277 | doi = 10.1038/nrn3111 | quote = ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure<sup>14,22–24</sup>. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption<sup>14,26–30</sup>. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states. }}</ref><ref name="Natural and drug addictions" /><ref name="ΔFosB reward"/> in particular, ΔFosB in the nucleus accumbens is critical for the [[reinforcing]] effects of sexual reward.<ref name="ΔFosB reward" /> Research on the interaction between natural and drug rewards suggests that dopaminergic psychostimulants (e.g., [[amphetamine]]) and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess bidirectional cross-[[sensitization]] effects that are mediated through ΔFosB.<ref name="Natural and drug addictions" /><ref name="Amph-Sex X-sensitization through D1 signaling"><!--Supplemental primary source—>{{cite journal |vauthors=Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM | title = Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator |journal=[[The Journal of Neuroscience]] |volume=33 | issue = 8 | pages = 3434–42 |date=February 2013 | pmid = 23426671 | pmc = 3865508 | doi = 10.1523/JNEUROSCI.4881-12.2013 | quote = Drugs of abuse induce neuroplasticity in the natural reward pathway, specifically the nucleus accumbens (NAc), thereby causing development and expression of addictive behavior. ... Together, these findings demonstrate that drugs of abuse and natural reward behaviors act on common molecular and cellular mechanisms of plasticity that control vulnerability to drug addiction, and that this increased vulnerability is mediated by ΔFosB and its downstream transcriptional targets. ... Sexual behavior is highly rewarding (Tenk et al., 2009), and sexual experience causes sensitized drug-related behaviors, including cross-sensitization to amphetamine (Amph)-induced locomotor activity (Bradley and Meisel, 2001; Pitchers et al., 2010a) and enhanced Amph reward (Pitchers et al., 2010a). Moreover, sexual experience induces neural plasticity in the NAc similar to that induced by psychostimulant exposure, including increased dendritic spine density (Meisel and Mullins, 2006; Pitchers et al., 2010a), altered glutamate receptor trafficking, and decreased synaptic strength in prefrontal cortex-responding NAc shell neurons (Pitchers et al., 2012). Finally, periods of abstinence from sexual experience were found to be critical for enhanced Amph reward, NAc spinogenesis (Pitchers et al., 2010a), and glutamate receptor trafficking (Pitchers et al., 2012). These findings suggest that natural and drug reward experiences share common mechanisms of neural plasticity}}</ref><ref name="Amph-Sex X-sensitization through NMDA signaling"><!--Supplemental primary source—>{{cite journal | vauthors = Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM | title = Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats | journal = Neuropharmacology | volume = 101 | issue = | pages = 154–64 | date = February 2016 | pmid = 26391065 | doi = 10.1016/j.neuropharm.2015.09.023 | quote = }}</ref> This phenomenon is notable since, in humans, a [[dopamine dysregulation syndrome]], characterized by drug-induced compulsive engagement in natural rewards (specifically, sexual activity, shopping, and gambling), has also been observed in some individuals taking [[dopaminergic]] medications.<ref name="Natural and drug addictions" />
| + | Addiction to both drugs and behavioral rewards may arise from similar dysregulation of the mesolimbic dopamine system. Preclinical evidence has demonstrated that marked increases in the expression of ΔFosB through repetitive and excessive exposure to a natural reward induces the same behavioral effects and [[neuroplasticity]] as occurs in a drug addiction.<ref name="Natural and drug addictions" /> |
| | | | |
| − | [[ΔFosB]] inhibitors (drugs or treatments that oppose its action) may be an effective treatment for addiction and addictive disorders.<ref name="Malenka_2009_04">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 384–85 | edition = 2nd | chapter = Chapter 15: Reinforcement and addictive disorders }}</ref> | + | ===Psychiatric and medical classifications=== |
| | + | Behavioral addictions were introduced as a new diagnostic category in [[DSM-5]], but only [[gambling]] addiction is included. Internet gaming addiction is included in the appendix as a condition for further study. Diagnostic models do not currently include the criteria necessary to identify behaviors as addictions in a clinical setting. |
| | | | |
| − | The release of [[dopamine]] in the [[nucleus accumbens]] plays a role in the reinforcing qualities of many forms of stimuli, including naturally reinforcing stimuli like palatable food and sex.<ref name=Salamone>{{cite journal|last=Salamone|first=J.D.|title=Complex motor and sensorimotor function of striatal and accumbens dopamine: Involvement in instrumental behavior processes|journal=Psychopharmacology|year=1992|volume=107|issue=2–3|pages=160–74|doi=10.1007/bf02245133 |pmid=1615120}}</ref><ref name=Kauer>{{cite journal|last=Kauer|first=J.A.|author2=R.C. Malenka|title=Synaptic plasticity and addiction|journal=Nature Reviews Neuroscience |year=2007|issue=11|pages=844–58|doi=10.1038/nrn2234|pmid=17948030|volume=8}}</ref> Altered dopamine [[neurotransmission]] is frequently observed following the development of an addictive state.<ref name="Natural and drug addictions" /> In humans and lab animals that have developed an addiction, alterations in dopamine or [[opioid]] neurotransmission in the nucleus accumbens and other parts of the [[striatum]] are evident.<ref name="Natural and drug addictions" /> Studies have found that use of certain drugs (e.g., [[cocaine]]) affect [[cholinergic neuron]]s that innervate the [[reward system]], in turn affecting dopamine signaling in this region.<ref name=Witten>{{cite journal|last=Witten|first=I|author2=S.-C. Lin|author3=M Brodsky|title=Cholinergic interneurons control local circuit activity and cocaine conditioning|journal=Science|year=2010|volume=330|issue=6011|pages=1677–81|doi=10.1126/science.1193771|pmid=21164015|pmc=3142356|bibcode=2010Sci...330.1677W}}</ref>
| + | In September 2019, the [[American Society of Addiction Medicine]] (ASAM) issued a public statement defining all addiction in terms of brain changes: |
| | + | <blockquote>Addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences. People with addiction use substances or engage in behaviors that become compulsive and often continue despite harmful consequences.<ref name=ASAM/></blockquote> |
| | | | |
| − | ===Reward system===
| + | The type of excessive behaviors identified as being addictive include [[Problem gambling|gambling]], [[eating disorder|food]], [[Chocoholic|chocolate]], [[sexual addiction|sexual intercourse]], use of [[pornography addiction|pornography]], use of [[computer addiction|computers]], playing [[Video game addiction|video games]], use of the [[internet]] and other digital media, [[exercise]], and [[shopping]]. |
| − | {{main|Reward system}}
| |
| − | {{expand section|date=August 2015}}
| |
| | | | |
| − | ====Mesocorticolimbic pathway====
| + | [[Gambling]] provides a natural reward which is associated with compulsive behavior and for which clinical diagnostic manuals, namely the [[DSM-5]], have identified diagnostic criteria for an addiction. In order for a person's gambling behavior to meet criteria of an addiction, it shows certain characteristics, such as mood modification, compulsivity, and withdrawal. There is evidence from functional neuroimaging that gambling activates the reward system and the [[mesolimbic pathway]] in particular.<ref name="Behavioral addictions" /> Similarly, shopping and playing video games are associated with compulsive behaviors in humans and have also been shown to activate the mesolimbic pathway and other parts of the reward system.<ref name="Natural and drug addictions" /> Based upon this evidence, [[gambling addiction]], [[video game addiction]], and [[shopping addiction]] are classified accordingly.<ref name="Natural and drug addictions" /><ref name="Behavioral addictions" /> |
| − | {{Annotated image 4
| |
| − | | caption = Top: this depicts the initial effects of high dose exposure to an addictive drug on [[gene expression]] in the [[nucleus accumbens]] for various Fos family proteins (i.e., [[c-Fos]], [[FosB]], [[ΔFosB]], [[Fra1]], and [[Fra2]]).<br />Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these [[phosphorylated]] (35–37 [[kilodalton]]) ΔFosB [[isoform]]s persist in the [[D1-type]] [[medium spiny neurons]] of the nucleus accumbens for up to 2 months.<!--The following named ref is transcluded in from "Template:Psychostimulant addiction"—><ref name="Nestler2" /><ref name="pmid11572966">{{cite journal |vauthors=Nestler EJ, Barrot M, Self DW | title = DeltaFosB: a sustained molecular switch for addiction | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 98 | issue = 20 | pages = 11042–46 |date=September 2001 | pmid = 11572966 | pmc = 58680 | doi = 10.1073/pnas.191352698 | quote = Although the ΔFosB signal is relatively long-lived, it is not permanent. ΔFosB degrades gradually and can no longer be detected in brain after 1–2 months of drug withdrawal ... Indeed, ΔFosB is the longest-lived adaptation known to occur in adult brain, not only in response to drugs of abuse, but to any other perturbation (that doesn't involve lesions) as well. }}</ref>
| |
| − | | header = ΔFosB accumulation from excessive drug use
| |
| − | | header_background = aliceblue
| |
| − | | alt = ΔFosB accumulation graph
| |
| − | | image = ΔFosB accumulation.svg
| |
| − | | align = right
| |
| − | | icon = none
| |
| − | | image-width = 400
| |
| − | | image-left = 0
| |
| − | | image-top = 0
| |
| − | | width = 400
| |
| − | | height = 440
| |
| − | | annotations =
| |
| − | }}
| |
| − | Understanding the pathways in which drugs act and how drugs can alter those pathways is key when examining the biological basis of drug addiction. The reward pathway, known as the [[mesolimbic system|mesolimbic pathway]], or its extension, the [[mesocorticolimbic pathway]], is characterized by the interaction of several areas of the brain.
| |
| − | * The projections from the [[ventral tegmentum|ventral tegmental area]] (VTA) are a network of [[dopaminergic]] [[neurons]] with [[wikt:colocalize|co-localized]] postsynaptic [[glutamate]] receptors ([[AMPAR]] and [[NMDAR]]). These cells respond when stimuli indicative of a reward are present. The VTA supports learning and sensitization development and releases DA into the [[forebrain]].<ref name="Jones and Bonci">{{cite journal |vauthors=Jones S, Bonci A |title=Synaptic plasticity and drug addiction |journal=Current Opinion in Pharmacology |volume=5 |issue=1 |pages=20–25 |year=2005 |pmid=15661621 |doi=10.1016/j.coph.2004.08.011}}</ref> These neurons also project and release DA into the nucleus accumbens,<ref name="Eisch and Harburg">{{cite journal |vauthors=Eisch AJ, Harburg GC |title=Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology |journal=Hippocampus |volume=16 |issue=3 |pages=271–86 |year=2006 |pmid=16411230 |doi=10.1002/hipo.20161}}</ref> through the [[mesolimbic pathway]]. Virtually all drugs causing drug addiction increase the dopamine release in the mesolimbic pathway,<ref name=Rang>{{cite book |author=Rang, H.P. |title=Pharmacology |publisher=Churchill Livingstone |location=Edinburgh |year=2003 |page=596 |isbn=978-0-443-07145-4}}</ref> in addition to their specific effects.
| |
| − | * The [[nucleus accumbens]] (NAcc) is one output of the VTA projections. The nucleus accumbens itself consists mainly of [[GABA]]ergic [[medium spiny neuron]]s (MSNs).<ref name="Kourrich">{{cite journal |vauthors=Kourrich S, Rothwell PE, Klug JR, Thomas MJ |title=Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens |journal=J. Neurosci. |volume=27 |issue=30 |pages=7921–28 |year=2007 |pmid=17652583 |pmc=6672735 |doi=10.1523/JNEUROSCI.1859-07.2007}}</ref> The NAcc is associated with acquiring and eliciting conditioned behaviors, and is involved in the increased sensitivity to drugs as addiction progresses.<ref name="Jones and Bonci"/> Overexpression of [[ΔFosB]] in the nucleus accumbens is a necessary common factor in essentially all known forms of addiction;<ref name="Cellular basis" /> ΔFosB is a strong positive modulator of [[positively reinforced]] behaviors.<ref name="Cellular basis" />
| |
| − | * The [[prefrontal cortex]], including the [[anterior cingulate]] and [[orbitofrontal cortex|orbitofrontal]] cortices,<ref name="Kalivas and Volkow">{{cite journal | vauthors = Kalivas PW, Volkow ND | title = The neural basis of addiction: a pathology of motivation and choice | journal = The American Journal of Psychiatry | volume = 162 | issue = 8 | pages = 1403–13 | date = August 2005 | pmid = 16055761 | doi = 10.1176/appi.ajp.162.8.1403 }}</ref> is another VTA output in the mesocorticolimbic pathway; it is important for the integration of information which helps determine whether a behavior will be elicited.<ref name="Floresco">{{cite journal | vauthors = Floresco SB, Ghods-Sharifi S | title = Amygdala-prefrontal cortical circuitry regulates effort-based decision making | journal = Cerebral Cortex | volume = 17 | issue = 2 | pages = 251–60 | date = February 2007 | pmid = 16495432 | doi = 10.1093/cercor/bhj143 | citeseerx = 10.1.1.335.4681 }}</ref> It is also critical for forming associations between the rewarding experience of drug use and cues in the environment. Importantly, these cues are strong mediators of drug-seeking behavior and can trigger relapse even after months or years of abstinence.<ref>{{cite journal | vauthors = Perry CJ, Zbukvic I, Kim JH, Lawrence AJ | title = Role of cues and contexts on drug-seeking behaviour | journal = British Journal of Pharmacology | volume = 171 | issue = 20 | pages = 4636–72 | date = October 2014 | pmid = 24749941 | pmc = 4209936 | doi = 10.1111/bph.12735 }}</ref>
| |
| − | Other brain structures that are involved in addiction include:
| |
| − | * The [[basolateral amygdala]] projects into the NAcc and is thought to also be important for motivation.<ref name="Floresco"/>
| |
| − | * The [[hippocampus]] is involved in drug addiction, because of its role in learning and memory. Much of this evidence stems from investigations showing that manipulating cells in the hippocampus alters dopamine levels in NAcc and firing rates of VTA dopaminergic cells.<ref name="Eisch and Harburg"/>
| |
| | | | |
| − | ====Role of dopamine and glutamate====
| + | Reviews of both clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form of [[sexual intercourse]] – as an addiction. Moreover, [[#Reward sensitization|reward cross-sensitization]] between [[amphetamine]] and sexual activity, meaning that exposure to one increases the desire for both, has been shown to occur preclinically and clinically as a [[dopamine dysregulation syndrome]]; ΔFosB [[gene expression|expression]] is required for this cross-sensitization effect, which intensifies with the level of ΔFosB expression.<ref name="Natural and drug addictions" /> |
| | | | |
| − | Dopamine is the primary neurotransmitter of the reward system in the brain. It plays a role in regulating movement, emotion, cognition, motivation, and feelings of pleasure.<ref name=arch>{{cite journal | vauthors = Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F | title = Dopamine in drug abuse and addiction: results of imaging studies and treatment implications | journal = Arch. Neurol. | volume = 64 | issue = 11 | pages = 1575–79 | year = 2007 | pmid = 17998440 | doi = 10.1001/archneur.64.11.1575 | url = | doi-access = free }}</ref> Natural rewards, like eating, as well as recreational drug use cause a release of dopamine, and are associated with the reinforcing nature of these stimuli.<ref name=arch/><ref name=drugs-brain>{{cite web|url=http://www.drugabuse.gov/publications/science-addiction/drugs-brain|title=Drugs, Brains, and Behavior: The Science of Addiction|publisher=National Institute on Drug Abuse}}</ref> Nearly all addictive drugs, directly or indirectly, act upon the brain's reward system by heightening dopaminergic activity.<ref name=addict>{{cite web|url=http://www.drugabuse.gov/infofacts/understand.html|title=Understanding Drug Abuse and Addiction|publisher=National Institute on Drug Abuse|date= November 2012}}</ref>
| + | Reviews of preclinical studies indicate that long-term frequent and excessive consumption of high fat or sugar foods can produce an addiction ([[food addiction]]).<ref name="Natural and drug addictions" /> |
| | | | |
| − | Excessive intake of many types of addictive drugs results in repeated release of high amounts of dopamine, which in turn affects the reward pathway directly through heightened [[dopamine receptor]] activation. Prolonged and abnormally high levels of dopamine in the [[synaptic cleft]] can induce receptor [[Downregulation and upregulation|downregulation]] in the neural pathway. Downregulation of [[mesolimbic]] dopamine receptors can result in a decrease in the sensitivity to natural reinforcers.<ref name=arch/> | + | Excessive and compulsive [[Internet]] use has also been studied, revealing it to be a behavioral addiction with serious psychosocial consequences: |
| | + | <blockquote>The growing number of researches on Internet addiction indicates that Internet addiction is a psychosocial disorder and its characteristics are as follows: tolerance, withdrawal symptoms, affective disorders, and problems in social relations. Internet usage creates psychological, social, school and/or work difficulties in a person's life. Eighteen percent of study participants were considered to be pathological Internet users, whose excessive use of the Internet was causing academic, social, and interpersonal problems. Excessive Internet use may create a heightened level of psychological arousal, resulting in little sleep, failure to eat for long periods, and limited physical activity, possibly leading to the user experiencing physical and mental health problems such as depression, OCD, low family relationships and anxiety.<ref>Seyyed Salman Alavi, Mohammad Reza Maracy, Fereshte Jannatifard, and Mehdi Eslami, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3214398/ The effect of psychiatric symptoms on the internet addiction disorder in Isfahan's University students] ''J Res Med Sci.'' 16(6) (2011):793–800. Retrieved August 31, 2022.</ref></blockquote> |
| | | | |
| − | Drug seeking behavior is induced by glutamatergic projections from the prefrontal cortex to the nucleus accumbens. This idea is supported with data from experiments showing that drug seeking behavior can be prevented following the inhibition of [[AMPA]] glutamate receptors and glutamate release in the nucleus accumbens.<ref name="Kalivas and Volkow"/>
| + | Studies on Internet addiction reveal the same fundamental brain changes seen in other addictions.<ref>Fuchun Lin, Yan Zhou, Yasong Du, Lindi Qin, Zhimin Zhao, Jianrong Xu, and Hao Lei, [https://pubmed.ncbi.nlm.nih.gov/22253926/ Abnormal White Matter Integrity in Adolescents with Internet Addiction Disorder: A Tract-Based Spatial Statistics Study] ''PLoS One'' 7(1) (2012). Retrieved August 31, 2022.</ref><ref>Sang Hee Kim, Sang-Hyun Baik, Chang Soo Park, Su Jin Kim, Sung Won Choi, and Sang Eun Kim, [https://pubmed.ncbi.nlm.nih.gov/21499141/ Reduced Striatal Dopamine D2 Receptors in People With Internet Addiction] ''Neuroreport'' 22(8) (2011):407-411. Retrieved August 31, 2022.</ref> |
| | | | |
| − | ===Reward sensitization{{anchor|Sensitization|Drug sensitization}}===<!--"Drug sensitization" redirects here, do not change without correcting the redirect—>
| + | Another growing area is [[social media addiction]]. Researchers found that not only is [[social media]] (particularly [[Facebook]]) itself potentially addictive, those who use it may also be at greater risk for substance abuse.<ref>[https://www.albany.edu/news/56604.php Craving Facebook? UAlbany Study Finds Social Media to be Potentially Addictive, Associated with Substance Abuse] News Center, University at Albany, State University of New York, December 9, 2014. Retrieved August 31, 2022.</ref> |
| − | {| class="wikitable" style="text-align:center; float:right; margin-left:1em;"
| |
| − | |+ Neural and behavioral effects of validated ΔFosB transcriptional targets in the [[striatum]]<ref name="What the ΔFosB?" /><ref name="pmid18640924">{{cite journal |author=Nestler EJ |title=Review. Transcriptional mechanisms of addiction: role of DeltaFosB |journal=Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences |volume=363 |issue=1507 |pages=3245–55 |date=October 2008 |pmid=18640924 |doi=10.1098/rstb.2008.0067 |url= |pmc=2607320|quote=Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch – from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure – cited earlier (Renthal et al. in press). The mechanism responsible for ΔFosB repression of c-fos expression is complex and is covered below. ...<br />Examples of validated targets for ΔFosB in nucleus accumbens ... GluR2 ... dynorphin ... Cdk5 ... NFκB ... c-Fos}}<br />[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2607320/table/tbl3/ Table 3]</ref>
| |
| − | ! scope="col" | Target<br />gene
| |
| − | ! scope="col" | Target<br />[[gene expression|expression]]
| |
| − | ! scope="col" | Neural effects
| |
| − | ! scope="col" | Behavioral effects
| |
| − | |-
| |
| − | | scope="row" style="height: 40px"| [[c-Fos]] || ↓ || Molecular switch enabling the chronic<br />induction of ΔFosB{{#tag:ref|In other words, c-Fos [[gene repression|repression]] allows ΔFosB to more rapidly accumulate within the D1-type medium spiny neurons of the nucleus accumbens because it is selectively induced in this state.<ref name="Cellular basis" /> Prior to c-Fos repression, all Fos family proteins (e.g., c-Fos, [[Fra1]], [[Fra2]], [[FosB]], and ΔFosB) are induced together, with ΔFosB expression increasing to a lesser extent.<ref name="Cellular basis" />|group="note"}} || –
| |
| − | |-
| |
| − | | scope="row" style="height: 40px"| [[dynorphin]] || ↓<br />{{#tag:ref|According to two medical reviews, ΔFosB has been implicated in causing both increases and decreases in dynorphin expression in different studies;<ref name="What the ΔFosB?" /><ref name="pmid18640924" /> this table entry reflects only a decrease. |group="note"}} || style="text-align:left" | {{bull}}Downregulation of [[κ-opioid receptor|κ-opioid]] feedback loop || style="text-align:left" | {{bull}}Increased drug reward
| |
| − | |-
| |
| − | | scope="row" style="height: 40px"| [[NF-κB]] || ↑ || style="text-align:left" | {{bull}}Expansion of [[Nucleus accumbens|NAcc]] dendritic processes<br />{{bull}}NF-κB inflammatory response in the {{abbr|NAcc|nucleus accumbens}}<br />{{bull}}NF-κB inflammatory response in the {{abbrlink|CP|caudate putamen}}|| style="text-align:left" | {{bull}}Increased drug reward<br />{{bull}}Increased drug reward<br />{{bull}}[[Stereotypy|Locomotor sensitization]]
| |
| − | |-
| |
| − | | scope="row" style="height: 40px"| [[GluR2]] || ↑ || style="text-align:left" | {{bull}}Decreased [[sensitization|sensitivity]] to [[glutamate]] || style="text-align:left" | {{bull}}Increased drug reward
| |
| − | |-
| |
| − | | scope="row" style="height: 40px"| [[Cdk5]] || ↑ || style="text-align:left" | {{bull}}[[GluR1]] synaptic protein phosphorylation<br />{{bull}}Expansion of {{abbr|NAcc|nucleus accumbens}} dendritic processes || Decreased drug reward<br /><small>(net effect)</small>
| |
| − | |}
| |
| − | '''Reward sensitization''' is a process that causes an increase in the amount of reward (specifically, [[incentive salience]]{{#tag:ref|Incentive salience, the "[[motivational salience]]" for a reward, is a "desire" or "want" attribute, which includes a motivational component, that the brain assigns to a rewarding stimulus.<ref name="Incentive salience and motivation review" /><ref name="NAcc function" /> As a consequence, incentive salience acts as a motivational "magnet" for a rewarding stimulus that commands attention, induces approach, and causes the rewarding stimulus to be sought out.<ref name="Incentive salience and motivation review">{{cite journal | vauthors = Berridge KC | title = From prediction error to incentive salience: mesolimbic computation of reward motivation | journal = Eur. J. Neurosci. | volume = 35 | issue = 7 | pages = 1124–43 | date = April 2012 | pmid = 22487042 | pmc = 3325516 | doi = 10.1111/j.1460-9568.2012.07990.x | quote = Here I discuss how mesocorticolimbic mechanisms generate the motivation component of incentive salience. Incentive salience takes Pavlovian learning and memory as one input and as an equally important input takes neurobiological state factors (e.g. drug states, appetite states, satiety states) that can vary independently of learning. Neurobiological state changes can produce unlearned fluctuations or even reversals in the ability of a previously learned reward cue to trigger motivation. Such fluctuations in cue-triggered motivation can dramatically depart from all previously learned values about the associated reward outcome. ... Associative learning and prediction are important contributors to motivation for rewards. Learning gives incentive value to arbitrary cues such as a Pavlovian conditioned stimulus (CS) that is associated with a reward (unconditioned stimulus or UCS). Learned cues for reward are often potent triggers of desires. For example, learned cues can trigger normal appetites in everyone, and can sometimes trigger compulsive urges and relapse in addicts.<br />Cue-triggered ‘wanting’ for the UCS<br />A brief CS encounter (or brief UCS encounter) often primes a pulse of elevated motivation to obtain and consume more reward UCS. This is a signature feature of incentive salience.<br />Cue as attractive motivational magnets<br />When a Pavlovian CS+ is attributed with incentive salience it not only triggers ‘wanting’ for its UCS, but often the cue itself becomes highly attractive – even to an irrational degree. This cue attraction is another signature feature of incentive salience ... Two recognizable features of incentive salience are often visible that can be used in neuroscience experiments: (i) UCS-directed ‘wanting’ – CS-triggered pulses of intensified ‘wanting’ for the UCS reward; and (ii) CS-directed ‘wanting’ – motivated attraction to the Pavlovian cue, which makes the arbitrary CS stimulus into a motivational magnet.}}</ref>|group="note"}}) that is assigned by the brain to a rewarding stimulus (e.g., a drug). In simple terms, when reward sensitization to a specific stimulus (e.g., a drug) occurs, an individual's "wanting" or desire for the stimulus itself and its associated [[cue reactivity|cues]] increases.<ref name="NAcc function">{{cite book |vauthors=Malenka RC, Nestler EJ, Hyman SE |veditors=Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 147–48, 366–67, 375–76 | edition = 2nd | quote= VTA DA neurons play a critical role in motivation, reward-related behavior (Chapter 15), attention, and multiple forms of memory. This organization of the DA system, wide projection from a limited number of cell bodies, permits coordinated responses to potent new rewards. Thus, acting in diverse terminal fields, dopamine confers motivational salience ("wanting") on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). In this example, dopamine modulates the processing of sensorimotor information in diverse neural circuits to maximize the ability of the organism to obtain future rewards. ...<br />The brain reward circuitry that is targeted by addictive drugs normally mediates the pleasure and strengthening of behaviors associated with natural reinforcers, such as food, water, and sexual contact. Dopamine neurons in the VTA are activated by food and water, and dopamine release in the NAc is stimulated by the presence of natural reinforcers, such as food, water, or a sexual partner. ...<br />The NAc and VTA are central components of the circuitry underlying reward and memory of reward. As previously mentioned, the activity of dopaminergic neurons in the VTA appears to be linked to reward prediction. The NAc is involved in learning associated with reinforcement and the modulation of motoric responses to stimuli that satisfy internal homeostatic needs. The shell of the NAc appears to be particularly important to initial drug actions within reward circuitry; addictive drugs appear to have a greater effect on dopamine release in the shell than in the core of the NAc. ... If motivational drive is described in terms of wanting, and hedonic evaluation in terms of liking, it appears that wanting can be dissociated from liking and that dopamine may influence these phenomena differently. Differences between wanting and liking are confirmed in reports by human addicts, who state that their desire for drugs (wanting) increases with continued use even when pleasure (liking) decreases because of tolerance.}}</ref><ref name="Incentive salience and motivation review" /><ref name="Reinforcement in addiction" /> Reward sensitization normally occurs following chronically high levels of exposure to the stimulus. [[ΔFosB]] (DeltaFosB) expression in [[D1-type]] [[medium spiny neuron]]s in the [[nucleus accumbens]] has been shown to directly and positively regulate reward sensitization involving drugs and natural rewards.<ref name="Cellular basis" /><ref name="What the ΔFosB?" /><ref name="G9a reverses ΔFosB plasticity" />
| |
| | | | |
| − | "Cue-induced wanting" or "cue-triggered wanting", a form of craving that occurs in addiction, is responsible for most of the compulsive behavior that addicts exhibit.<ref name="Incentive salience and motivation review" /><ref name="Reinforcement in addiction">{{Cite book | vauthors = Edwards S | title = Reinforcement principles for addiction medicine; from recreational drug use to psychiatric disorder | journal = Prog. Brain Res. | volume = 223 | issue = | pages = 63–76 | year = 2016 | pmid = 26806771 | doi = 10.1016/bs.pbr.2015.07.005 | quote = An important dimension of reinforcement highly relevant to the addiction process (and particularly relapse) is secondary reinforcement (Stewart, 1992). Secondary reinforcers (in many cases also considered conditioned reinforcers) likely drive the majority of reinforcement processes in humans. In the specific case of drug addition, cues and contexts that are intimately and repeatedly associated with drug use will often themselves become reinforcing ... A fundamental piece of Robinson and Berridge's incentive-sensitization theory of addiction posits that the incentive value or attractive nature of such secondary reinforcement processes, in addition to the primary reinforcers themselves, may persist and even become sensitized over time in league with the development of drug addiction (Robinson and Berridge, 1993).| series = Progress in Brain Research | isbn = 978-0-444-63545-7 }}</ref> During the development of an addiction, the repeated association of otherwise neutral and even non-rewarding [[stimulation|stimuli]] with drug consumption triggers an [[associative learning]] process that causes these previously neutral stimuli to act as [[Reinforcement#Secondary reinforcers|conditioned positive reinforcers]] of addictive drug use (i.e., these stimuli start to function as [[drug cues]]).<ref name="Incentive salience and motivation review" /><ref name="Pleasure system - incentive sensitization">{{cite journal | vauthors = Berridge KC, Kringelbach ML | title = Pleasure systems in the brain | journal = Neuron | volume = 86 | issue = 3 | pages = 646–64 | date = May 2015 | pmid = 25950633 | doi = 10.1016/j.neuron.2015.02.018 | pmc=4425246}}</ref><ref name="Reinforcement in addiction" /> As conditioned positive reinforcers of drug use, these previously neutral stimuli are assigned incentive salience (which manifests as a craving) – sometimes at pathologically high levels due to reward sensitization – which can [[Pavlovian-instrumental transfer|transfer to the primary reinforcer]] (e.g., the use of an addictive drug) with which it was originally paired.<ref name="Incentive salience and motivation review" /><ref name="Pleasure system - incentive sensitization" /><ref name="Reinforcement in addiction" />
| + | ===Treatment=== |
| − | | + | Behavioral addiction is a treatable condition.<ref>Jon Grant, ''Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions'' (W. W. Norton & Company, 2008, ISBN 978-0393705218).</ref> Treatment options include [[psychotherapy]] and [[psychopharmacology|psychopharmacotherapy]] (medications) or a combination of both. [[Cognitive behavioral therapy]] (CBT) is the most common form of psychotherapy used in treating behavioral addictions; it focuses on identifying patterns that trigger [[compulsive behavior]] and making lifestyle changes to promote healthier behaviors. While CBT does not cure behavioral addiction, it does help with coping with the condition in a healthy way. |
| − | Research on the interaction between natural and drug rewards suggests that dopaminergic psychostimulants (e.g., [[amphetamine]]) and sexual behavior act on similar biomolecular mechanisms to induce ΔFosB in the nucleus accumbens and possess a bidirectional '''reward cross-sensitization''' effect{{#tag:ref|In simplest terms, this means that when either amphetamine or sex is perceived as more alluring or desirable through reward sensitization, this effect occurs with the other as well.|group="note"}} that is mediated through ΔFosB.<ref name="Natural and drug addictions" /><ref name="Amph-Sex X-sensitization through D1 signaling" /><ref name="Amph-Sex X-sensitization through NMDA signaling" /> In contrast to ΔFosB's reward-sensitizing effect, [[CREB]] transcriptional activity decreases user's sensitivity to the rewarding effects of the substance. CREB transcription in the nucleus accumbens is implicated in [[psychological dependence]] and symptoms involving a [[anhedonia|lack of pleasure or motivation]] during [[drug withdrawal]].<ref name="Cellular basis" /><ref name="pmid11572966" /><ref name="pmid18640924" />
| |
| − | | |
| − | The set of proteins known as "[[regulators of G protein signaling]]" (RGS), particularly [[RGS4]] and [[RGS9-2]], have been implicated in modulating some forms of opioid sensitization, including reward sensitization.<ref name="RGS opioid">{{cite journal | vauthors = Traynor J | title = μ-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology | journal = Drug Alcohol Depend | volume = 121 | issue = 3 | pages = 173–80 | date = March 2012 | pmid = 22129844 | pmc = 3288798 | doi = 10.1016/j.drugalcdep.2011.10.027 | quote = }}</ref>
| |
| − | {{clear}}
| |
| − | {{Addiction-related plasticity|title=Summary of addiction-related plasticity}}
| |
| − | {{clear}}
| |
| − | | |
| − | ===Neuroepigenetic mechanisms===
| |
| − | {{See also|Neuroepigenetics|Chromatin remodeling}}
| |
| − | {{Expand section|the table of drug-induced chromatin modifications from figure 2 of this<ref name="Chromatin states" /> review; an explanation of the relationship between class I [[HDAC]]s—[[H3K9ac]]/[[H3K9ac2]], [[G9a]]—[[H3K9me2]], and transcriptional mechanisms (i.e., phospho-CREB and FosB–ΔFosB)|date=June 2018|small=no}}<!--
| |
| − | -Should probably mention the following since it's related to both the mechanisms and research sections:
| |
| − | 1) Prolonged inhibition of class I HDACs results in histone hyper-acetylation, which in turn increases G9a expression and H3K9me2 synthesis
| |
| − | 2) G9a catalyzes H3K9 dimethylation (H3K9me2 synthesis)
| |
| − | 3) H3K9me2 is a repressive epigenetic mark that inhibits the induction of DeltaFosB in the NAcc by preventing transcription of the FosB gene
| |
| − | i.e., +HDACi → ↑ H3 acetylation & ↑H4 acetylation → ↑G9a → ↑H3K9me2 synthesis
| |
| − | 4) The feedback loop involving: +drug → ↑ΔFosB → ↓G9a → ↓H3K9me2 → ↑ΔFosB
| |
| − | —>
| |
| − | Altered [[epigenetic]] regulation of [[gene expression]] within the brain's reward system plays a significant and complex role in the development of drug addiction.<ref name="Nestler 2014 epigenetics" /><ref name="Chromatin states" /> Addictive drugs are associated with three types of epigenetic modifications within neurons.<ref name="Nestler 2014 epigenetics" /> These are (1) [[histone modification]]s, (2) [[epigenetic methylation]] of DNA at [[CpG site]]s at (or adjacent to) particular genes, and (3) epigenetic [[Downregulation and upregulation|downregulation or upregulation]] of [[microRNA]]s which have particular target genes.<ref name="Nestler 2014 epigenetics" /><ref name="Nestler" /><ref name="Chromatin states" /> As an example, while hundreds of genes in the cells of the nucleus accumbens (NAc) exhibit histone modifications following drug exposure – particularly, altered acetylation and methylation states of [[histone]] [[Residue (chemistry)#Biochemistry|residues]]<ref name="Chromatin states" /> – most other genes in the NAc cells do not show such changes.<ref name="Nestler 2014 epigenetics" />
| |
| − | | |
| − | ==Diagnosis==
| |
| − | {{Further|Substance use disorder#Diagnosis|Problem gambling#Diagnosis}}
| |
| − | The 5th edition of the [[Diagnostic and Statistical Manual of Mental Disorders]] (DSM-5) uses the term "[[substance use disorder]]" to refer to a spectrum of drug use-related disorders. The DSM-5 eliminates the terms "[[drug abuse|abuse]]" and "dependence" from diagnostic categories, instead using the specifiers of ''mild'', ''moderate'' and ''severe'' to indicate the extent of disordered use. These specifiers are determined by the number of diagnostic criteria present in a given case. In the DSM-5, the term ''drug addiction'' is synonymous with ''severe substance use disorder''.<ref name="Surgeon General severe SUD" /><ref name="Brain disease" />
| |
| − | | |
| − | The DSM-5 introduced a new diagnostic category for behavioral addictions; however, [[problem gambling]] is the only condition included in that category in the 5th edition.<ref name="Addiction-dependence distinction" /> [[Internet gaming disorder]] is listed as a "condition requiring further study" in the DSM-5.<ref>{{cite journal | vauthors = Petry NM, Rehbein F, Gentile DA, Lemmens JS, Rumpf HJ, Mößle T, Bischof G, Tao R, Fung DS, Borges G, Auriacombe M, González Ibáñez A, Tam P, O'Brien CP | title = An international consensus for assessing internet gaming disorder using the new DSM-5 approach | journal = Addiction | volume = 109 | issue = 9 | pages = 1399–406 | date = September 2014 | pmid = 24456155 | doi = 10.1111/add.12457 }}</ref>
| |
| − | | |
| − | Past editions have used [[physical dependence]] and the associated withdrawal syndrome to identify an addictive state. [[Physical dependence]] occurs when the body has adjusted by incorporating the substance into its "normal" functioning – i.e., attains [[homeostasis]] – and therefore physical withdrawal symptoms occur upon cessation of use.<ref name="pmid10511013">{{cite journal |vauthors=Torres G, Horowitz JM | title = Drugs of abuse and brain gene expression | journal = Psychosom Med | volume = 61 | issue = 5 | pages = 630–50 | year = 1999 | pmid = 10511013 | doi = 10.1097/00006842-199909000-00007| citeseerx = 10.1.1.326.4903 }}</ref> Tolerance is the process by which the body continually adapts to the substance and requires increasingly larger amounts to achieve the original effects. Withdrawal refers to physical and psychological symptoms experienced when reducing or discontinuing a substance that the body has become dependent on. Symptoms of withdrawal generally include but are not limited to body aches, [[anxiety]], [[irritability]], intense [[craving (withdrawal)|cravings]] for the substance, [[nausea]], [[hallucinations]], [[headaches]], cold sweats, [[tremor]]s, and seizures.
| |
| − | | |
| − | Medical researchers who actively study addiction have criticized the DSM classification of addiction for being flawed and involving arbitrary diagnostic criteria.<ref name="NHMH_3e terms-DSM flaw">{{cite book | vauthors = Malenka RC, Nestler EJ, Hyman SE, Holtzman DM | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2015 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-182770-6 | edition = 3rd | chapter = Chapter 16: Reinforcement and Addictive Disorders | quote = The official diagnosis of drug addiction by the Diagnostic and Statistic Manual of Mental Disorders (2013), which uses the term substance use disorder, is flawed. Criteria used to make the diagnosis of substance use disorders include tolerance and somatic dependence/withdrawal, even though these processes are not integral to addiction as noted. It is ironic and unfortunate that the manual still avoids use of the term addiction as an official diagnosis, even though addiction provides the best description of the clinical syndrome.}}</ref> Writing in 2013, the director of the United States [[National Institute of Mental Health]] discussed the invalidity of the DSM-5's classification of mental disorders:<ref name="DSM-V Insel">{{cite web|author1=Thomas Insel|title=Transforming Diagnosis|url=http://www.nimh.nih.gov/about/director/2013/transforming-diagnosis.shtml|publisher=National Institute of Mental Health|accessdate=17 June 2015}}</ref>
| |
| − | <blockquote> While DSM has been described as a "Bible" for the field, it is, at best, a dictionary, creating a set of labels and defining each. The strength of each of the editions of DSM has been "reliability" – each edition has ensured that clinicians use the same terms in the same ways. The weakness is its lack of validity. Unlike our definitions of ischemic heart disease, lymphoma, or AIDS, the DSM diagnoses are based on a consensus about clusters of clinical symptoms, not any objective laboratory measure. In the rest of medicine, this would be equivalent to creating diagnostic systems based on the nature of chest pain or the quality of fever.</blockquote>
| |
| − | | |
| − | Given that addiction manifests in structural changes to the brain, it is possible that non-invasive [[neuroimaging]] scans obtained via [[MRI]] could be used to help diagnose addiction in the future.<ref name="Addiction and Brain Structure">{{cite journal | vauthors = Hampton WH, Hanik I, Olson IR | title = Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research | language = English | journal = Drug and Alcohol Dependence | volume = 197 | issue = 4 | pages = 288–298 | year = 2019 | pmid = 30875650 | pmc = 6440853 | doi = 10.1016/j.drugalcdep.2019.02.005 | quote = Despite this progress, our ability to predict, diagnose, and track addiction in humans based on brain images has been relatively limited. The difficulty elucidating such outcomes may be partly due to a relative dearth of research considering neural white matter, which constitutes over half of human brain volume and plays a vital role in governing communication between cortical areas (Fields, 2008). Diffusion mag- netic resonance imaging has emerged as a method to non-invasively examine white matter in the human brain and relate such connectivity to substance abuse and addictive behaviors (Suckling and Nestor, 2017)}}</ref> As a diagnostic [[biomarker (medicine)|biomarker]], [[ΔFosB]] expression could be used to diagnose an addiction in humans, but this would require a [[brain biopsy]] and therefore is not used in clinical practice.
| |
| − | | |
| − | ==Treatment==
| |
| − | {{see also|Addiction recovery groups|Cognitive behavioral therapy|Drug rehabilitation}}
| |
| − | According to a review, "in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, [[twelve-step program]]s, and residential treatment facilities."<ref name="Reward system and psychostimulants" />
| |
| − | | |
| − | ===Behavioral therapy===
| |
| − | A meta-analytic review on the efficacy of various [[Behavioral therapy|behavioral therapies]] for treating drug and behavioral addictions found that [[cognitive behavioral therapy]] (e.g., [[relapse prevention]] and [[contingency management]]), [[motivational interviewing]], and a [[Community reinforcement approach and family training|community reinforcement approach]] were effective interventions with moderate effect sizes.<ref name="German meta-analysis">{{cite journal | vauthors = Walter M, Dürsteler KM, Petitjean SA, Wiesbeck GA, Euler S, Sollberger D, Lang UE, Vogel M | title = [Psychosocial Treatment of Addictive Disorders – An Overview of Psychotherapeutic Options and their Efficacy] | language = German | journal = Fortschr Neurol Psychiatr | volume = 83 | issue = 4 | pages = 201–10 | year = 2015 | pmid = 25893493 | doi = 10.1055/s-0034-1399338 | quote = Addictive disorders are chronic relapsing conditions marked by compulsive and often uncontrolled use of psychotropic substances or stimuli. In this review, we present and discuss the current specific psychosocial interventions for addictive disorders and their effectiveness. In particular cognitive behavioral therapy, motivational interviewing, relapse prevention, the community reinforcement approach, and contingency management were found to be effective. For these psychotherapeutic treatments, mostly moderate effect sizes have been found. Their effectiveness seems to be highest in cannabis dependence. Empirical evidence for dependence on "hard" drugs is largest for contingency management, while for alcohol dependence motivational interviewing and the community reinforcement approach show the largest effect sizes. Presumably, combinations of different approaches as well as online interventions will bring further progress in the psychosocial treatment of addictive disorders in the future.}}</ref>
| |
| − | | |
| − | Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (e.g., [[marathon running]]), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and for psychostimulant addiction in particular.<ref name="Natural and drug addictions" /><ref name="Addiction review 2016">{{cite journal | vauthors = Carroll ME, Smethells JR | title = Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments | journal = Front. Psychiatry | volume = 6 | issue = | pages = 175 | date = February 2016 | pmid = 26903885 | pmc = 4745113 | doi = 10.3389/fpsyt.2015.00175 | quote = Environmental Enrichment ...<br />In humans, non-drug rewards delivered in a contingency management (CM) format successfully reduced drug dependence ... In general, CM programs promote drug abstinence through a combination of positive reinforcement for drug-free urine samples. For instance, voucher-based reinforcement therapy in which medication compliance, therapy session attendance, and negative drug screenings reinforced with vouchers to local business (e.g., movie theater, restaurants, etc.) directly reinforces drug abstinence, provides competing reinforcers, enriches the environment, and it is a robust treatment across a broad range of abused drugs (189). ...<br />Physical Exercise<br />There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... However, a <abbr title="randomized controlled trial">RTC</abbr> study was recently reported by Rawson et al. (226), whereby they used 8 weeks of exercise as a post-residential treatment for METH addiction, showed a significant reduction in use (confirmed by urine screens) in participants who had been using meth 18 days or less a month. ... '''Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon.''' [emphasis added]}}</ref><ref name="Running vs addiction">{{cite journal |vauthors=Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA | title = Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis | journal = Neurosci Biobehav Rev | volume = 37 | issue = 8 | pages = 1622–44 |date=September 2013 | pmid = 23806439 | pmc = 3788047 | doi = 10.1016/j.neubiorev.2013.06.011 | quote = [exercise] efficacy may be related to its ability to normalize glutamatergic and dopaminergic signaling and reverse drug-induced changes in chromatin via epigenetic interactions with brain-derived neurotrophic factor (BDNF) in the reward pathway. ... these data show that exercise can affect dopaminergic signaling at many different levels, which may underlie its ability to modify vulnerability during drug use initiation. Exercise also produces neuroadaptations that may influence an individual's vulnerability to initiate drug use. Consistent with this idea, chronic moderate levels of forced treadmill running blocks not only subsequent methamphetamine-induced conditioned place preference, but also stimulant-induced increases in dopamine release in the NAc (Chen et al., 2008) and striatum (Marques et al., 2008). ... [These] findings indicate the efficacy of exercise at reducing drug intake in drug-dependent individuals ... wheel running [reduces] methamphetamine self-administration under extended access conditions (Engelmann et al., 2013) ... These findings suggest that exercise may "magnitude"-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuro-adaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes (see Table 4). ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.}}</ref><ref name="Exercise Rev 3" /><ref name="Exercise, addiction prevention, and ΔFosB">{{cite journal | vauthors = Zhou Y, Zhao M, Zhou C, Li R | title = Sex differences in drug addiction and response to exercise intervention: From human to animal studies | journal = Front. Neuroendocrinol. | volume = 40| issue = | pages = 24–41| date = July 2015 | pmid = 26182835 | doi = 10.1016/j.yfrne.2015.07.001 | quote = Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... As briefly reviewed above, a large number of human and rodent studies clearly show that there are sex differences in drug addiction and exercise. The sex differences are also found in the effectiveness of exercise on drug addiction prevention and treatment, as well as underlying neurobiological mechanisms. The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation. ... In particular, more studies on the neurobiological mechanism of exercise and its roles in preventing and treating drug addiction are needed. | pmc=4712120}}</ref> Consistent aerobic exercise magnitude-dependently (i.e., by duration and intensity) reduces drug addiction risk, which appears to occur through the reversal of drug induced addiction-related neuroplasticity.<ref name="Natural and drug addictions" /><ref name="Running vs addiction" /> One review noted that exercise may prevent the development of drug addiction by altering [[ΔFosB]] or {{nowrap|[[c-Fos]]}} [[immunoreactivity]] in the [[striatum]] or other parts of the [[reward system]].<ref name="Exercise, addiction prevention, and ΔFosB" /> Aerobic exercise decreases drug self-administration, reduces the likelihood of relapse, and induces opposite effects on [[striatum|striatal]] [[dopamine receptor D2|dopamine receptor D<sub>2</sub>]] (DRD2) signaling (increased DRD2 density) to those induced by addictions to several drug classes (decreased DRD2 density).<ref name="Natural and drug addictions" /><ref name="Running vs addiction" /> Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.<ref name="Natural and drug addictions" /><ref name="Running vs addiction" /><ref name="Exercise Rev 3">{{cite journal | vauthors = Linke SE, Ussher M | title = Exercise-based treatments for substance use disorders: evidence, theory, and practicality | journal = Am J Drug Alcohol Abuse | volume = 41 | issue = 1 | pages = 7–15 | year = 2015 | pmid = 25397661 | doi = 10.3109/00952990.2014.976708 | quote = The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects. | pmc=4831948}}</ref>
| |
| − | | |
| − | ===Medication===
| |
| − | | |
| − | ====Alcohol addiction====
| |
| − | {{further|Alcoholism}}
| |
| − | Alcohol, like opioids, can induce a severe state of [[physical dependence]] and produce withdrawal symptoms such as [[delirium tremens]]. Because of this, treatment for alcohol addiction usually involves a combined approach dealing with dependence and addiction simultaneously. Benzodiazepines have the largest and the best evidence base in the treatment of alcohol withdrawal and are considered the gold standard of [[alcohol detoxification]].<ref>Sachdeva A, Choudhary M, Chandra M. (Sep 2015) [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4606320/ "Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond."] [[PMID]]: [https://www.ncbi.nlm.nih.gov/pubmed/26500991 26500991]</ref>
| |
| − | | |
| − | Pharmacological treatments for alcohol addiction include drugs like [[naltrexone]] (opioid antagonist), [[disulfiram]], [[acamprosate]], and [[topiramate]].<ref>{{cite journal | vauthors = Soyka M, Roesner S | title = New pharmacological approaches for the treatment of alcoholism | journal = Expert Opinion on Pharmacotherapy | volume = 7 | issue = 17 | pages = 2341–53 | date = December 2006 | pmid = 17109610 | doi = 10.1517/14656566.7.17.2341 }}</ref><ref>{{cite journal | vauthors = Pettinati HM, Rabinowitz AR | title = Choosing the right medication for the treatment of alcoholism | journal = Current Psychiatry Reports | volume = 8 | issue = 5 | pages = 383–88 | date = October 2006 | pmid = 16968619 | doi = 10.1007/s11920-006-0040-0 }}</ref> Rather than substituting for alcohol, these drugs are intended to affect the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.<ref>{{cite journal | vauthors = Bouza C, Angeles M, Magro A, Muñoz A, Amate JM | title = Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review | journal = Addiction | volume = 99 | issue = 7 | pages = 811–28 | date = July 2004 | pmid = 15200577 | doi = 10.1111/j.1360-0443.2004.00763.x }}</ref><ref>{{cite journal | vauthors = Williams SH | title = Medications for treating alcohol dependence | journal = American Family Physician | volume = 72 | issue = 9 | pages = 1775–80 | date = November 2005 | pmid = 16300039 }}</ref> According to a [[Cochrane Collaboration]] review, the opioid antagonist [[naltrexone]] has been shown to be an effective treatment for alcoholism, with the effects lasting three to twelve months after the end of treatment.<ref>{{cite journal | vauthors = Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M | title = Opioid antagonists for alcohol dependence | journal = The Cochrane Database of Systematic Reviews | issue = 12 | pages = CD001867 | date = December 2010 | pmid = 21154349 | doi = 10.1002/14651858.CD001867.pub2 | editor1-last = Srisurapanont | editor1-first = Manit }}</ref>
| |
| − | | |
| − | ====Behavioral addictions====
| |
| − | {{transcluded section|source=Behavioral addiction}}
| |
| − | {{trim|{{#section-h:Behavioral addiction|Treatment}}}}
| |
| − | | |
| − | ====Cannabinoid addiction====
| |
| − | | |
| − | {{As of|2010}}, there are no effective pharmacological interventions for cannabinoid addiction.<ref name="Marijuana addiction 2010 review">{{cite journal | vauthors = Sofuoglu M, Sugarman DE, Carroll KM | title = Cognitive function as an emerging treatment target for marijuana addiction | journal = Exp Clin Psychopharmacol | volume = 18 | issue = 2 | pages = 109–19 | date = April 2010 | pmid = 20384422 | pmc = 2909584 | doi = 10.1037/a0019295 | quote = Cannabis is the most widely used illicit substance in the world, and demand for effective treatment is increasing. However, abstinence rates following behavioral therapies have been modest, and there are no effective pharmacotherapies for the treatment of cannabis addiction.}}</ref> A 2013 review on cannabinoid addiction noted that the development of [[CB1 receptor]] agonists that have reduced interaction with [[β-arrestin 2]] signaling might be therapeutically useful.<ref name="Cannabinoid addiction 2013 review">{{cite journal | vauthors = Fratta W, Fattore L | title = Molecular mechanisms of cannabinoid addiction | journal = Curr. Opin. Neurobiol. | volume = 23 | issue = 4 | pages = 487–92 | date = August 2013 | pmid = 23490548 | doi = 10.1016/j.conb.2013.02.002 | quote = 14. Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ: b-Arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region dependent manner. Biol Psychiatry 2012, 71:714–24.<br />A pioneering study revealing both positive and negative modulatory effects of beta-arrestin2 on THC tolerance. By demonstrating that tolerance to antinociception is reduced whereas tolerance to catalepsy is enhanced in beta-arrestin2 knockout mice, authors suggest that development of cannabinoid agonists that minimize interactions between CB1Rs and beta-arrestin2 might produce improved cannabinoid analgesics with reduced motor suppression, and be therapeutically beneficial.}}</ref>
| |
| − | | |
| − | ====Nicotine addiction====
| |
| − | {{Further|Smoking cessation}}
| |
| − | Another area in which drug treatment has been widely used is in the treatment of [[nicotine]] addiction, which usually involves the use of [[nicotine replacement therapy]], [[nicotinic receptor antagonist]]s, or [[nicotinic receptor]] [[partial agonist]]s.<ref name="nicotine addiction therapies" /><ref name="pmid24484986" /> Examples of drugs that act on nicotinic receptors and have been used for treating nicotine addiction include antagonists like [[bupropion]] and the partial agonist [[varenicline]].<ref name="nicotine addiction therapies">{{cite journal |vauthors=Garwood CL, Potts LA |title=Emerging pharmacotherapies for smoking cessation |journal=Am J Health Syst Pharm |volume= 64 |issue=16 |pages=1693–98 |year=2007 |pmid=17687057 |doi=10.2146/ajhp060427}}</ref><ref name="pmid24484986">{{Cite book | vauthors = Crooks PA, Bardo MT, Dwoskin LP | title = Nicotinic receptor antagonists as treatments for nicotine abuse | journal = Adv. Pharmacol. | volume = 69 | issue = | pages = 513–51 | year = 2014 | pmid = 24484986 | pmc = 4110698 | doi = 10.1016/B978-0-12-420118-7.00013-5 | url = | series = Advances in Pharmacology | isbn = 978-0-12-420118-7 }}</ref>
| |
| − | | |
| − | ====Opioid addiction====
| |
| − | {{Further|Opioid use disorder}}
| |
| − | Opioids cause [[physical dependence]], and treatment typically addresses both dependence and addiction.
| |
| − | | |
| − | Physical dependence is treated using replacement drugs such as [[suboxone]] or [[subutex]] (both containing the active ingredients [[buprenorphine]]) and [[methadone]].<ref>{{cite journal |vauthors=Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE |title=A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence |journal=N. Engl. J. Med. |volume=343 |issue=18 |pages=1290–97 |year=2000 |pmid=11058673|doi=10.1056/NEJM200011023431802}}</ref><ref>{{cite journal |vauthors=Connock M, Juarez-Garcia A, Jowett S |title=Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation |journal= Health Technol Assess |volume=11 |issue=9 |pages=1–171, iii–iv |year=2007 |pmid=17313907|display-authors=etal |doi=10.3310/hta11090|doi-access=free }}</ref> Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a measure of control over both pain and cravings. Use of replacement drugs increases the addicted individual's ability to function normally and eliminates the negative consequences of obtaining controlled substances illicitly. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases. In the United States, opiate replacement therapy is tightly regulated in [[methadone clinic]]s and under the [[DATA 2000]] legislation. In some countries, other opioid derivatives such as [[levomethadyl acetate]],<ref>{{cite journal |vauthors=Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE |title=Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence |journal=Exp Clin Psychopharmacol |volume=13 |issue=4 |pages=293–302 |year=2005 |pmid=16366759 |doi=10.1037/1064-1297.13.4.293}}</ref> [[dihydrocodeine]],<ref>{{cite journal |vauthors=Robertson JR, Raab GM, Bruce M, McKenzie JS, Storkey HR, Salter A |title=Addressing the efficacy of dihydrocodeine versus methadone as an alternative maintenance treatment for opiate dependence: A randomized controlled trial |journal=[[Addiction (journal)|Addiction]] |volume=101 |issue=12 |pages=1752–59 |year=2006 |pmid=17156174 |doi=10.1111/j.1360-0443.2006.01603.x}}</ref> [[dihydroetorphine]]<ref>{{cite journal |author=Qin Bo-Yi |title=Advances in dihydroetorphine: From analgesia to detoxification |journal=Drug Development Research|volume=39|issue=2 |pages=131–34 |year=1998 |doi= 10.1002/(SICI)1098-2299(199610)39:2<131::AID-DDR3>3.0.CO;2-Q}} [https://archive.today/20121210055522/http://www3.interscience.wiley.com/cgi-bin/abstract/67067/ABSTRACT?CRETRY=1&SRETRY=0 Link]</ref> and even [[heroin]]<ref name="Metrebian1">{{cite journal |vauthors=Metrebian N, Shanahan W, Wells B, Stimson GV |title=Feasibility of prescribing injectable heroin and methadone to opiate-dependent drug users: associated health gains and harm reductions |journal=Med. J. Aust. |volume=168 |issue=12 |pages=596–600 |year=1998 |pmid=9673620|doi=10.5694/j.1326-5377.1998.tb141444.x }}</ref><ref name="Metrebian2">{{cite journal |vauthors=Metrebian N, Mott J, Carnwath Z, Carnwath T, Stimson GV, Sell L |title=Pathways into receiving a prescription for diamorphine (heroin) for the treatment of opiate dependence in the United kingdom |journal=Eur Addict Res |volume=13 |issue=3 |pages=144–47 |year=2007 |pmid=17570910 |doi=10.1159/000101550}}</ref> are used as substitute drugs for illegal street opiates, with different prescriptions being given depending on the needs of the individual patient. [[Baclofen]] has led to successful reductions of cravings for stimulants, alcohol, and opioids, and also alleviates [[alcohol withdrawal syndrome]]. Many patients have stated they "became indifferent to alcohol" or "indifferent to cocaine" overnight after starting baclofen therapy.<ref name="Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM 2007 213–37">{{cite journal |vauthors=Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM |title=Pharmacotherapy of dual substance abuse and dependence |journal=CNS Drugs |volume=21 |issue=3 |pages=213–37 |year=2007 |pmid=17338593 |doi= 10.2165/00023210-200721030-00003}}</ref> Some studies show the interconnection between opioid [[drug detoxification]] and overdose mortality.<ref>Strang J, McCambridge J. (May 2003) [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC153851/ "Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study"] [[PMID]]: [https://www.ncbi.nlm.nih.gov/pubmed/12727768 12727768]</ref>
| |
| − | | |
| − | ====Psychostimulant addiction====
| |
| − | {{As of|May 2014}}, there is no effective [[pharmacotherapy]] for any form of psychostimulant addiction.<ref name="Reward system and psychostimulants">{{cite journal | vauthors = Taylor SB, Lewis CR, Olive MF | title = The neurocircuitry of illicit psychostimulant addiction: acute and chronic effects in humans | journal = Subst. Abuse Rehabil. | volume = 4 | pages = 29–43 | date = February 2013 | pmid = 24648786 | pmc = 3931688 | doi = 10.2147/SAR.S39684 | quote = Initial drug use can be attributed to the ability of the drug to act as a reward (ie, a pleasurable emotional state or positive reinforcer), which can lead to repeated drug use and dependence.<sup>8,9</sup> A great deal of research has focused on the molecular and neuroanatomical mechanisms of the initial rewarding or reinforcing effect of drugs of abuse. ... At present, no pharmacological therapy has been approved by the FDA to treat psychostimulant addiction. Many drugs have been tested, but none have shown conclusive efficacy with tolerable side effects in humans.<sup>172</sup> ...<!--These drugs have included DA-receptor ligands, such as DA receptor agonists, partial agonists, and antagonists, as well as DA-reuptake inhibitors.<sup>173,174</sup> One newer dopaminergic drug that has shown some promise is the antipsychotic medication aripiprazole, a partial agonist at D2-like receptors, which is currently approved for the treatment of schizophrenia, depression, and bipolar disorder.<sup>175</sup> Clinical trials have thus far been mixed, with one study finding reduced cocaine craving and use, and another study showing increased cocaine use.<sup>176,177</sup> ...
| |
| − | | |
| − | Recent evidence for dysregulation of glutamatergic signaling in addiction has led to the testing of N-acetylcysteine, a derivative of the amino acid cysteine that normalizes extracellular levels of Glu following cocaine administration.<sup>178,179</sup> Clinical trials found that N-acetylcysteine treatment attenuates cocaine craving and use and normalizes brain glutamate levels.<sup>180–182</sup> Other recent studies found trends toward significant reductions in METH use and craving produced by the antidepressant buproprion and the anticonvulsant topiramate, which has glutamate release-inhibiting properties.<sup>183,184</sup> While these compounds have not demonstrated overwhelming efficacy in reducing psychostimulant use or craving in all subjects tested, trends toward effects were observed and thus merit further investigation.
| |
| − | | |
| − | Other compounds that have been tested, with disappointing results, include β-adrenergic antagonists, opioid-receptor antagonists, 5-HT3-receptor antagonists, antidepressants, and anticonvulsants.<sup>172,185–190</sup> Despite the lack of past successes, several newer medications are currently being investigated and have shown some initial success, such as the smoking-cessation aid varenicline, the α2 adrenergic agonist clonidine, and the antidepressant mirtazapine.<sup>191–194</sup> In addition, preclinical evidence strongly suggests potential therapeutic effects of compounds targeting receptors for endocannabinoids, the neuropeptide orexin, and CRF for the prevention of relapse to psychostimulant use.<sup>195–197</sup>
| |
| − | | |
| − | Future approaches for the treatment of psychostimulant-induced cognitive deficits and dependence include the use of cognitive enhancers.<sup>198–200</sup> Two promising examples include prescription stimulants, such as modafinil and methylphenidate, which have shown modest but potential treatment effects in METH users and cocaine users with comorbid attention deficit/hyperactivity disorder, respectively.<sup>201–205</sup> In addition, galantamine, a cholinergic modulator and cognitive enhancer, has been reported to both decrease cocaine use and improve sustained attention in dependent subjects.<sup>206,207</sup> Together, these studies indicate that clinical trials of cognitive enhancers in the treatment of psychostimulant addiction are warranted. In addition to the pharmacological trials reviewed above, less conventional strategies that are gaining scientific momentum include vaccine therapies to immunoneutralize drug molecules and impede penetrance across the blood–brain barrier, enzyme conjugates that dramatically increase the metabolic breakdown of abused drugs, pharmacogenetic approaches based on individual genetic polymorphisms in addiction-related genes, and epigenetic modulators of drug-induced changes in gene expression.<sup>208–211</sup> While still in their relative infancy, these exciting new avenues of research offer a significant expansion of possible biologically based targets for the treatment of psychostimulant addiction. ...
| |
| − | | |
| − | The tremendous need for more effective pharmacological treatments for psychostimulant addiction is a mainstay of contemporary addiction research. However, the recent downsizing of many major pharmaceutical companies away from psychiatric indications (including addiction) due to the lack of efficacy of experimental compounds in humans may require a sea change in the translational research approach.<sup>212,213</sup> —> A new emphasis on larger-scale biomarker, genetic, and epigenetic research focused on the molecular targets of mental disorders has been recently advocated.<sup>212</sup> In addition, the integration of cognitive and behavioral modification of circuit-wide neuroplasticity (ie, computer-based training to enhance executive function) may prove to be an effective adjunct-treatment approach for addiction, particularly when combined with cognitive enhancers.<sup>198,213–216</sup> Furthermore, in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities.}}</ref><ref name="pmid24716825">{{cite journal | vauthors = Stoops WW, Rush CR | title = Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research | journal = Expert Rev Clin Pharmacol | volume = 7 | issue = 3 | pages = 363–74 | date = May 2014 | pmid = 24716825 | doi = 10.1586/17512433.2014.909283 | quote = Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved. | pmc=4017926}}</ref><ref name="Cochrane 2013 treatments">{{cite journal | vauthors = Perez-Mana C, Castells X, Torrens M, Capella D, Farre M | title = Efficacy of psychostimulant drugs for amphetamine abuse or dependence | journal = Cochrane Database Syst. Rev. | volume = 9 | issue = 9| pages = CD009695 | date = September 2013 | pmid = 23996457 | doi = 10.1002/14651858.CD009695.pub2 | quote = To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. ... Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy}}</ref><ref name="pmid23039267">{{cite journal | vauthors = Forray A, Sofuoglu M | title = Future pharmacological treatments for substance use disorders | journal = Br. J. Clin. Pharmacol. | volume = 77 | issue = 2 | pages = 382–400 | date = February 2014 | pmid = 23039267 | pmc = 4014020 | doi = 10.1111/j.1365-2125.2012.04474.x }}</ref> Reviews from 2015, 2016, and 2018 indicated that [[TAAR1]]-[[selective agonist]]s have significant therapeutic potential as a treatment for psychostimulant addictions;<ref name="Miller+Grandy 2016" /><ref name="TAAR1 addiction 2015" /><ref name="Curable brain disorder?">{{cite journal | vauthors = Liu JF, Li JX | title = Drug addiction: a curable mental disorder? | journal = Acta Pharmacologica Sinica | volume = 39 | issue = 12 | pages = 1823–1829 | date = December 2018 | pmid = 30382181 | pmc = 6289334 | doi = 10.1038/s41401-018-0180-x | url = }}</ref> however, {{As of|2018|lc=y}}, the only compounds which are known to function as TAAR1-selective agonists are [[experimental drug]]s.<ref name="Miller+Grandy 2016">{{cite journal | vauthors = Grandy DK, Miller GM, Li JX | title = "TAARgeting Addiction" – The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference | journal = Drug Alcohol Depend. | volume = 159 | issue = | pages = 9–16 | date = February 2016 | pmid = 26644139 | doi = 10.1016/j.drugalcdep.2015.11.014 | quote = When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse. | pmc=4724540}}</ref><ref name="TAAR1 addiction 2015">{{cite journal | vauthors = Jing L, Li JX | title = Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction | journal = Eur. J. Pharmacol. | volume = 761 | issue = | pages = 345–52 | date = August 2015 | pmid = 26092759 | doi = 10.1016/j.ejphar.2015.06.019 | quote = Taken together, the data reviewed here strongly support that TAAR1 is implicated in the functional regulation of monoaminergic systems, especially dopaminergic system, and that TAAR1 serves as a homeostatic "brake" system that is involved in the modulation of dopaminergic activity. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction. ... Given that TAAR1 is primarily located in the intracellular compartments and existing TAAR1 agonists are proposed to get access to the receptors by translocation to the cell interior (Miller, 2011), future drug design and development efforts may need to take strategies of drug delivery into consideration (Rajendran et al., 2010). | pmc=4532615}}</ref><ref name="Curable brain disorder?" />
| |
| − | | |
| − | ====Research====
| |
| − | {{expand section|date=April 2016}}
| |
| − | Research indicates that vaccines which utilize anti-drug [[monoclonal antibodies]] can mitigate drug-induced positive reinforcement by preventing the drug from moving across the [[blood–brain barrier]];<ref name="Vaccine-antibody immunotherapy">{{cite journal | vauthors = Zalewska-Kaszubska J | title = Is immunotherapy an opportunity for effective treatment of drug addiction? | journal = Vaccine | volume = 33 | issue = 48 | pages = 6545–51 | date = November 2015 | pmid = 26432911 | doi = 10.1016/j.vaccine.2015.09.079 | url = }}</ref> however, current vaccine-based therapies are only effective in a relatively small subset of individuals.<ref name="Vaccine-antibody immunotherapy" /><ref name="Primary source: Antibody level limitation">{{cite journal | vauthors = Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD, Titcombe PJ, Mueller DL, Tubo NJ, Griffith TS, Pravetoni M | title = The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse | journal = J. Immunol. | volume = 194 | issue = 12 | pages = 5926–36 | date = June 2015 | pmid = 25972483 | pmc = 4458396 | doi = 10.4049/jimmunol.1500385 | quote = Translation of therapeutic vaccines for addiction, cancer, or other chronic noncommunicable diseases has been slow because only a small subset of immunized subjects achieved effective Ab levels.}}</ref> {{As of|November 2015}}, vaccine-based therapies are being tested in human clinical trials as a treatment for addiction and preventive measure against drug overdoses involving nicotine, cocaine, and methamphetamine.<ref name="Vaccine-antibody immunotherapy" />
| |
| − | | |
| − | The new study shows, that the vaccine may also save lives during a [[drug overdose]]. In this instance, the idea is that the body will respond to the vaccine by quickly producing antibodies to prevent the opioids from accessing the brain.<ref>Painter A. (2019) [https://vtnews.vt.edu/articles/2019/09/cals-mikezhang.html "Researchers working to develop vaccines to fight opioid addiction"] ''Vtnews.vt.edu''</ref>
| |
| − | | |
| − | Since addiction involves abnormalities in [[glutamate (neurotransmitter)|glutamate]] and [[GABAergic]] neurotransmission,<ref name="ATS pharmacotherapies" /><ref name="Glutamate and GABA systems" /> receptors associated with these neurotransmitters (e.g., [[AMPA receptor]]s, [[NMDA receptor]]s, and [[GABAB receptor|GABA<sub>B</sub> receptors]]) are potential therapeutic targets for addictions.<ref name="ATS pharmacotherapies">{{cite journal | vauthors = Cao DN, Shi JJ, Hao W, Wu N, Li J | title = Advances and challenges in pharmacotherapeutics for amphetamine-type stimulants addiction | journal = Eur. J. Pharmacol. | volume = 780| issue = | pages = 129–35| date = March 2016 | pmid = 27018393 | doi = 10.1016/j.ejphar.2016.03.040 | quote = }}</ref><ref name="Glutamate and GABA systems">{{cite journal | vauthors = Moeller SJ, London ED, Northoff G | title = Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity | journal = Neurosci Biobehav Rev | volume = 61 | issue = | pages = 35–52 | date = February 2016 | pmid = 26657968 | pmc = 4731270 | doi = 10.1016/j.neubiorev.2015.11.010 | url = }}</ref><ref name="GABAB PAMs">{{cite journal | vauthors = Agabio R, Colombo G | title = [GABAB receptor as therapeutic target for drug addiction: from baclofen to positive allosteric modulators] | language = Polish | journal = Psychiatr. Pol. | volume = 49 | issue = 2 | pages = 215–23 | date = April 2015 | pmid = 26093587 | doi = 10.12740/PP/33911 | url = | doi-access = free }}</ref><ref name="GABAB PAMs 2">{{cite journal | vauthors = Filip M, Frankowska M, Sadakierska-Chudy A, Suder A, Szumiec L, Mierzejewski P, Bienkowski P, Przegaliński E, Cryan JF | title = GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators | journal = Neuropharmacology | volume = 88 | issue = | pages = 36–47 | date = January 2015 | pmid = 24971600 | doi = 10.1016/j.neuropharm.2014.06.016 | quote = }}</ref> [[N-acetylcysteine]], which affects [[metabotropic glutamate receptor]]s and NMDA receptors, has shown some benefit in preclinical and clinical studies involving addictions to cocaine, heroin, and cannabinoids.<ref name="ATS pharmacotherapies" /> It may also be useful as an [[adjunct therapy]] for addictions to [[amphetamine-type stimulants]], but more clinical research is required.<ref name="ATS pharmacotherapies" />
| |
| − | | |
| − | Current medical reviews of research involving lab animals have identified a drug class – class I [[histone deacetylase inhibitor]]s{{#tag:ref|Inhibitors of class I [[histone deacetylase]] (HDAC) enzymes are drugs that inhibit four specific [[histone-modifying enzyme]]s: [[HDAC1]], [[HDAC2]], [[HDAC3]], and [[HDAC8]]. Most of the animal research with HDAC inhibitors has been conducted with four drugs: [[butyric acid|butyrate salts]] (mainly [[sodium butyrate]]), [[trichostatin A]], [[valproic acid]], and [[vorinostat|SAHA]];<ref name="Amphetamine epigenetics" /><ref name="Chromatin states" /> butyric acid is a naturally occurring [[short-chain fatty acid]] in humans, while the latter two compounds are FDA-approved drugs with [[medical indication]]s unrelated to addiction.|group="note"}} – that indirectly inhibits the function and further increases in the expression of accumbal ΔFosB by inducing [[G9a]] expression in the nucleus accumbens after prolonged use.<ref name="G9a reverses ΔFosB plasticity" /><ref name="Nestler 2014 epigenetics">{{cite journal | vauthors = Nestler EJ | title = Epigenetic mechanisms of drug addiction | journal = Neuropharmacology | volume = 76 Pt B | issue = | pages = 259–68 | date = January 2014 | pmid = 23643695 | pmc = 3766384 | doi = 10.1016/j.neuropharm.2013.04.004 | quote = Short-term increases in histone acetylation generally promote behavioral responses to the drugs, while sustained increases oppose cocaine's effects, based on the actions of systemic or intra-NAc administration of HDAC inhibitors. ... Genetic or pharmacological blockade of G9a in the NAc potentiates behavioral responses to cocaine and opiates, whereas increasing G9a function exerts the opposite effect (Maze et al., 2010; Sun et al., 2012a). Such drug-induced downregulation of G9a and H3K9me2 also sensitizes animals to the deleterious effects of subsequent chronic stress (Covington et al., 2011). Downregulation of G9a increases the dendritic arborization of NAc neurons, and is associated with increased expression of numerous proteins implicated in synaptic function, which directly connects altered G9a/H3K9me2 in the synaptic plasticity associated with addiction (Maze et al., 2010).<br />G9a appears to be a critical control point for epigenetic regulation in NAc, as we know it functions in two negative feedback loops. It opposes the induction of ΔFosB, a long-lasting transcription factor important for drug addiction (Robison and Nestler, 2011), while ΔFosB in turn suppresses G9a expression (Maze et al., 2010; Sun et al., 2012a). ... Also, G9a is induced in NAc upon prolonged HDAC inhibition, which explains the paradoxical attenuation of cocaine's behavioral effects seen under these conditions, as noted above (Kennedy et al., 2013). GABAA receptor subunit genes are among those that are controlled by this feedback loop. Thus, chronic cocaine, or prolonged HDAC inhibition, induces several GABAA receptor subunits in NAc, which is associated with increased frequency of inhibitory postsynaptic currents (IPSCs). In striking contrast, combined exposure to cocaine and HDAC inhibition, which triggers the induction of G9a and increased global levels of H3K9me2, leads to blockade of GABAA receptor and IPSC regulation. }}</ref><ref name="Amphetamine epigenetics">{{cite journal | vauthors = McCowan TJ, Dhasarathy A, Carvelli L | title = The Epigenetic Mechanisms of Amphetamine | journal = J. Addict. Prev. | volume = 2015 | issue = Suppl 1 | pages = | date = February 2015 | pmid = 27453897 | pmc = 4955852 | doi = | quote = Epigenetic modifications caused by addictive drugs play an important role in neuronal plasticity and in drug-induced behavioral responses. Although few studies have investigated the effects of AMPH on gene regulation (Table 1), current data suggest that AMPH acts at multiple levels to alter histone/DNA interaction and to recruit transcription factors which ultimately cause repression of some genes and activation of other genes. Importantly, some studies have also correlated the epigenetic regulation induced by AMPH with the behavioral outcomes caused by this drug, suggesting therefore that epigenetics remodeling underlies the behavioral changes induced by AMPH. If this proves to be true, the use of specific drugs that inhibit histone acetylation, methylation or DNA methylation might be an important therapeutic alternative to prevent and/or reverse AMPH addiction and mitigate the side effects generate by AMPH when used to treat ADHD.}}</ref><ref name="Chromatin states">{{cite journal | vauthors = Walker DM, Cates HM, Heller EA, Nestler EJ | title = Regulation of chromatin states by drugs of abuse | journal = Curr. Opin. Neurobiol. | volume = 30 | issue = | pages = 112–21 | date = February 2015 | pmid = 25486626 | doi = 10.1016/j.conb.2014.11.002 | quote = Studies investigating general HDAC inhibition on behavioral outcomes have produced varying results but it seems that the effects are specific to the timing of exposure (either before, during or after exposure to drugs of abuse) as well as the length of exposure | pmc=4293340}}</ref> These reviews and subsequent preliminary evidence which used [[oral administration]] or [[intraperitoneal administration]] of the sodium salt of [[butyric acid]] or other class I HDAC inhibitors for an extended period indicate that these drugs have efficacy in reducing addictive behavior in lab animals{{#tag:ref|Specifically, prolonged administration of a class I HDAC inhibitor appears to reduce an animal's motivation to acquire and use an addictive drug without affecting an animals motivation to attain other rewards (i.e., it does not appear to cause [[motivational anhedonia]]) and reduce the amount of the drug that is [[self-administration|self-administered]] when it is readily available.<ref name="Nestler 2014 epigenetics" /><ref name="Chromatin states" /><ref name="HDACi primaries" />|group="note"}} that have developed addictions to ethanol, psychostimulants (i.e., amphetamine and cocaine), nicotine, and opiates;<ref name="Nestler 2014 epigenetics" /><ref name="Chromatin states" /><ref name="HDACi primaries">Primary references involving sodium butyrate:<br />{{bull}}{{cite journal | vauthors = Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ | title = Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation | journal = Nat. Neurosci. | volume = 16 | issue = 4 | pages = 434–40 | date = April 2013 | pmid = 23475113 | pmc = 3609040 | doi = 10.1038/nn.3354 | quote = While acute HDAC inhibition enhances the behavioral effects of cocaine or amphetamine<sup>1,3,4,13,14</sup>, studies suggest that more chronic regimens block psychostimulant-induced plasticity<sup>3,5,11,12</sup>. ... The effects of pharmacological inhibition of HDACs on psychostimulant-induced plasticity appear to depend on the timecourse of HDAC inhibition. Studies employing co-administration procedures in which inhibitors are given acutely, just prior to psychostimulant administration, report heightened behavioral responses to the drug<sup>1,3,4,13,14</sup>. In contrast, experimental paradigms like the one employed here, in which HDAC inhibitors are administered more chronically, for several days prior to psychostimulant exposure, show inhibited expression<sup>3</sup> or decreased acquisition of behavioral adaptations to drug<sup>5,11,12</sup>. The clustering of seemingly discrepant results based on experimental methodologies is interesting in light of our present findings. Both HDAC inhibitors and psychostimulants increase global levels of histone acetylation in NAc. Thus, when co-administered acutely, these drugs may have synergistic effects, leading to heightened transcriptional activation of psychostimulant-regulated target genes. In contrast, when a psychostimulant is given in the context of prolonged, HDAC inhibitor-induced hyperacetylation, homeostatic processes may direct AcH3 binding to the promoters of genes (e.g., G9a) responsible for inducing chromatin condensation and gene repression (e.g., via H3K9me2) in order to dampen already heightened transcriptional activation. Our present findings thus demonstrate clear cross talk among histone PTMs and suggest that decreased behavioral sensitivity to psychostimulants following prolonged HDAC inhibition might be mediated through decreased activity of HDAC1 at H3K9 KMT promoters and subsequent increases in H3K9me2 and gene repression.}}<br />{{bull}}{{cite journal | vauthors = Simon-O'Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C | title = The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals | journal = Addict Biol | volume = 20 | issue = 4 | pages = 676–89 | date = July 2015 | pmid = 25041570 | doi = 10.1111/adb.12161 | quote = Altogether, our results clearly demonstrated the efficacy of {{abbr|NaB|sodium butyrate}} in preventing excessive ethanol intake and relapse and support the hypothesis that {{abbr|HDACi|HDAC inhibitors}} may have a potential use in alcohol addiction treatment.}}<br />{{bull}}{{cite journal | vauthors = Castino MR, Cornish JL, Clemens KJ | title = Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats | journal = PLOS One | volume = 10 | issue = 4 | pages = e0124796 | date = April 2015 | pmid = 25880762 | pmc = 4399837 | doi = 10.1371/journal.pone.0124796 | quote = treatment with NaB significantly attenuated nicotine and nicotine + cue reinstatement when administered immediately ... These results provide the first demonstration that HDAC inhibition facilitates the extinction of responding for an intravenously self-administered drug of abuse and further highlight the potential of HDAC inhibitors in the treatment of drug addiction.| bibcode = 2015PLoSO..1024796C }}</ref><ref name="HDAC alcoholism review">{{cite journal | vauthors = Kyzar EJ, Pandey SC | title = Molecular mechanisms of synaptic remodeling in alcoholism | journal = Neurosci. Lett. | volume = 601 | issue = | pages = 11–19 | date = August 2015 | pmid = 25623036 | pmc = 4506731 | doi = 10.1016/j.neulet.2015.01.051 | quote = Increased HDAC2 expression decreases the expression of genes important for the maintenance of dendritic spine density such as BDNF, Arc, and NPY, leading to increased anxiety and alcohol-seeking behavior. Decreasing HDAC2 reverses both the molecular and behavioral consequences of alcohol addiction, thus implicating this enzyme as a potential treatment target (Fig. 3). HDAC2 is also crucial for the induction and maintenance of structural synaptic plasticity in other neurological domains such as memory formation [115]. Taken together, these findings underscore the potential usefulness of HDAC inhibition in treating alcohol use disorders ... Given the ability of HDAC inhibitors to potently modulate the synaptic plasticity of learning and memory [118], these drugs hold potential as treatment for substance abuse-related disorders. ... Our lab and others have published extensively on the ability of HDAC inhibitors to reverse the gene expression deficits caused by multiple models of alcoholism and alcohol abuse, the results of which were discussed above [25,112,113]. This data supports further examination of histone modifying agents as potential therapeutic drugs in the treatment of alcohol addiction ... Future studies should continue to elucidate the specific epigenetic mechanisms underlying compulsive alcohol use and alcoholism, as this is likely to provide new molecular targets for clinical intervention.}}</ref> however, few clinical trials involving human addicts and any HDAC class I inhibitors have been conducted to test for treatment efficacy in humans or identify an optimal dosing regimen.{{#tag:ref|Among the few clinical trials that employed a class I HDAC inhibitor, one utilized [[valproate]] for methamphetamine addiction.<ref name="Valproate for methamphetamine addiction primary source">{{cite journal | vauthors = Kheirabadi GR, Ghavami M, Maracy MR, Salehi M, Sharbafchi MR | title = Effect of add-on valproate on craving in methamphetamine depended patients: A randomized trial | journal = Advanced Biomedical Research | volume = 5 | issue = | pages = 149 | date = 2016 | pmid = 27656618 | pmc = 5025910 | doi = 10.4103/2277-9175.187404 | quote = }}</ref>|group="note"}}
| |
| | | | |
| − | [[Gene therapy]] for addiction is an active area of research. One line of gene therapy research involves the use of [[viral vector]]s to increase the expression of [[dopamine D2 receptor]] proteins in the brain.<ref>{{cite web|url=https://www.sciencedaily.com/releases/2008/04/080416081628.htm|title=Gene Therapy For Addiction: Flooding Brain With 'Pleasure Chemical' Receptors Works On Cocaine, As On Alcohol}}</ref><ref>{{cite web|url=https://www.drugabuse.gov/news-events/nida-notes/2016/01/gene-transfer-therapy-cocaine-addiction-passes-tests-in-animals|title=Gene Transfer Therapy for Cocaine Addiction Passes Tests in Animals|first=National Institute on Drug|last=Abuse|date=14 January 2016}}</ref><ref>{{cite journal| pmc=4077905 | pmid=24892251 | doi=10.1016/j.vaccine.2014.05.067 | volume=32 | issue=33 | title=Physiologic and metabolic safety of butyrylcholinesterase gene therapy in mice | year=2014 | vauthors=Murthy V, Gao Y, Geng L, LeBrasseur NK, White TA, Parks RJ, Brimijoin S | journal=Vaccine | pages=4155–62}}</ref><ref>{{cite web|url=http://abstracts.aaps.org/Verify/AAPS2014/PosterSubmissions/T3009.pdf|title=Using Adeno-Associated Virus (AAV) Mediated Sustained Expression of an Anti-methamphetamine Antibody Fragment to Alter Methamphetamine Disposition in Mice }}</ref><ref>{{cite web|url=http://www.attcnetwork.org/explore/priorityareas/science/tools/asmeDetails.asp?ID=69|title=ATTC – Addiction Science Made Easy|website=www.attcnetwork.org}}</ref>
| + | Currently, there are no medications approved for treatment of behavioral addictions in general, but some medications used for treatment of drug addiction may also be beneficial with specific behavioral addictions.<ref name="Behavioral addictions">Jon E. Grant, Marc N. Potenza, Aviv Weinstein, and David A. Gorelick, [https://pubmed.ncbi.nlm.nih.gov/20560821/ Introduction to Behavioral Addictions] ''Am J Drug Alcohol Abuse'' 36(5) (2010):233-241. Retrieved August 31, 2022.</ref> Any unrelated psychiatric disorders should be kept under control, and differentiated from the contributing factors that cause the addiction. |
| | | | |
| | ==Epidemiology== | | ==Epidemiology== |
| − | | + | Due to cultural variations, the proportion of individuals who develop a drug or behavioral addiction within a specified time period (the [[prevalence]]) varies over time, by country, and across national [[demographics|population demographics]] (age group, socioeconomic status, and so forth).<ref name="Transgenerational epigenetic inheritance in addiction" /> |
| − | Due to cultural variations, the proportion of individuals who develop a drug or behavioral addiction within a specified time period (i.e., the [[prevalence]]) varies over time, by country, and across national [[demographics|population demographics]] (e.g., by age group, socioeconomic status, etc.).<ref name="Transgenerational epigenetic inheritance in addiction" /> | |
| | | | |
| | ===Asia=== | | ===Asia=== |
| − | The prevalence of alcohol dependence is not as high as is seen in other regions. In Asia, not only socioeconomic factors but also biological factors influence drinking behavior.<ref>Chen CC, Yin SJ. (Oct 2008) "Alcohol abuse and related factors in Asia". [[PMID]]: [https://www.ncbi.nlm.nih.gov/pubmed/19012127 19012127]</ref> | + | The prevalence of alcohol dependence is not as high as is seen in other regions. In Asia, not only socioeconomic factors but also biological factors influence drinking behavior.<ref>Chiao-Chicy Chen and Shih-Jiun Yin, [https://pubmed.ncbi.nlm.nih.gov/19012127/ Alcohol abuse and related factors in Asia] ''Int. Rev. Psychiatry'' 20(5) (2008):425-433. Retrieved August 31, 2022.</ref> |
| − | | |
| − | The overall prevalence of smartphone ownership is 62%, ranging from 41% in China to 84% in South Korea. Moreover, participation in online gaming ranges from 11% in China to 39% in Japan. Hong Kong has the highest number of adolescents reporting daily or above Internet use (68%). [[Internet addiction disorder]] is highest in the Philippines, according to both the IAT (Internet Addiction Test) – 5% and the CIAS-R (Revised Chen Internet Addiction Scale) – 21%.<ref>Mak KK, Lai CM, Watanabe H. (Nov 2014) "Epidemiology of internet behaviors and addiction among adolescents in six Asian countries". [[PMID]]: [https://www.ncbi.nlm.nih.gov/pubmed/25405785 25405785]</ref>
| |
| | | | |
| − | ===Australia=== | + | ===Europe and Oceania=== |
| − | The prevalence of substance abuse disorder among Australians was reported at 5.1% in 2009.<ref>{{Cite web | vauthors = Slade T, Johnston A, Teesson M, whiteford H, Burgess P, Pirkis J, Saw S | authorlink3= Maree Teesson | title = The Mental Health of Australians 2: Substance Use Disorders in Australia | publisher = Department of Health and Ageing, Canberra | date = May 2009 | url = https://www.health.gov.au/internet/main/publishing.nsf/Content/A24556C814804A99CA257BF0001CAC45/$File/mha26.pdf }}</ref>
| + | A 2017 report noted that [[Eastern Europe]] had the highest mortality rates for alcohol and illicit drugs, while [[Oceania]] had the highest [[tobacco]] mortality rates.<ref>Amy Peacock et al., [https://pubmed.ncbi.nlm.nih.gov/29749059/ Global statistics on alcohol, tobacco and illicit drug use: 2017 status report] ''Addiction'' 113(10) (2018):1905-1926. Retrieved August 31, 2022.</ref> |
| − | | |
| − | ===Europe===
| |
| − | In 2015, the estimated prevalence among the adult population was 18.4% for heavy episodic alcohol use (in the past 30 days); 15.2% for daily tobacco smoking; and 3.8, 0.77, 0.37 and 0.35% in 2017 cannabis, amphetamine, opioid and cocaine use. The mortality rates for alcohol and illicit drugs were highest in Eastern Europe.<ref>Peacock A, Leung J, Larney S. (Oct 2018) "Global statistics on alcohol, tobacco and illicit drug use: 2017 status report." [[PMID]]: [https://www.ncbi.nlm.nih.gov/pubmed/29749059 29749059]</ref>
| |
| | | | |
| | ===United States=== | | ===United States=== |
| | + | Addiction is widespread in the United States. According to a 2017 poll conducted by the [[Pew Research Center]], almost half of US adults know a family member or close friend who has struggled with a drug addiction at some point in their life.<ref>John Gramlich, [https://www.pewresearch.org/fact-tank/2017/10/26/nearly-half-of-americans-have-a-family-member-or-close-friend-whos-been-addicted-to-drugs/ Nearly half of Americans have a family member or close friend who's been addicted to drugs] ''Pew Research Center'', October 26, 2017. Retrieved August 31, 2022.</ref> |
| | | | |
| − | Based upon [[representative sample]]s of the US youth population in 2011, the [[lifetime prevalence]]{{#tag:ref|The lifetime prevalence of an addiction is the percentage of individuals in a population that developed an addiction at some point in their life.|group="note"}} of addictions to alcohol and illicit drugs has been estimated to be approximately 8% and 2–3% respectively.<ref name="Epidem" /> Based upon representative samples of the US adult population in 2011, the [[period prevalence|12 month prevalence]] of alcohol and illicit drug addictions were estimated at roughly 12% and 2–3% respectively.<ref name="Epidem" /> The lifetime prevalence of [[prescription drug]] addictions is currently around 4.7%.<ref>{{cite journal | vauthors = Manubay JM, Muchow C, Sullivan MA | title = Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics | journal = Primary Care | volume = 38 | issue = 1 | pages = 71–90 | date = March 2011 | pmid = 21356422 | pmc = 3328297 | doi = 10.1016/j.pop.2010.11.006 }}</ref>
| + | In spite of the massive overall economic cost to society, which is greater than the cost of [[diabetes]] and all forms of [[cancer]] combined, most doctors in the US lack the training to effectively address a drug addiction.<ref name="ABAM" /> In 2016, it was reported that only about ten percent, or a little over 2 million, receive any form of treatments, and those that do generally do not receive [[evidence-based medicine|evidence-based care]]. A major milestone was reached on March 14, 2016, when the American Board of Medical Specialties (ABMS) formally announced recognition of the field of Addiction Medicine as a medical subspecialty: <blockquote>This landmark event, more than any other, recognizes addiction as a preventable and treatable disease, helping to shed the stigma that has long plagued it. It sends a strong message to the public that American medicine is committed to providing expert care for this disease and services designed to prevent the risky substance use that precedes it.<ref name="NIDA Addiction Medicine">Nora Volkow, [https://archives.drugabuse.gov/about-nida/noras-blog/2016/03/major-step-forward-addiction-medicine A Major Step Forward for Addiction Medicine] ''National Institute on Drug Abuse'', March 31, 2016. Retrieved August 31, 2022.</ref></blockquote> |
| | | | |
| − | {{As of|2016|post=,}} about 22 million people in the United States need treatment for an addiction to alcohol, nicotine, or other drugs.<ref name="ABAM" /><ref name="NIDA Addiction Medicine" /> Only about 10%, or a little over 2 million, receive any form of treatments, and those that do generally do not receive [[evidence-based medicine|evidence-based care]].<ref name="ABAM">{{cite web|title=American Board of Medical Specialties recognizes the new subspecialty of addiction medicine|url=http://www.abam.net/wp-content/uploads/2016/03/1.-News-Release-ADM.pdf|website=American Board of Addiction Medicine|accessdate=3 April 2016|date=14 March 2016|quote= Sixteen percent of the non-institutionalized U.S. population age 12 and over – more than 40 million Americans – meets medical criteria for addiction involving nicotine, alcohol or other drugs. This is more than the number of Americans with cancer, diabetes or heart conditions. In 2014, 22.5 million people in the United States needed treatment for addiction involving alcohol or drugs other than nicotine, but only 11.6 percent received any form of inpatient, residential, or outpatient treatment. Of those who do receive treatment, few receive evidence-based care. (There is no information available on how many individuals receive treatment for addiction involving nicotine.)<br />Risky substance use and untreated addiction account for one-third of inpatient hospital costs and 20 percent of all deaths in the United States each year, and cause or contribute to more than 100 other conditions requiring medical care, as well as vehicular crashes, other fatal and non-fatal injuries, overdose deaths, suicides, homicides, domestic discord, the highest incarceration rate in the world and many other costly social consequences. The economic cost to society is greater than the cost of diabetes and all cancers combined. Despite these startling statistics on the prevalence and costs of addiction, few physicians have been trained to prevent or treat it.}}</ref><ref name="NIDA Addiction Medicine">{{cite web|author=Nora Volkow|title=A Major Step Forward for Addiction Medicine|url=https://www.drugabuse.gov/about-nida/noras-blog/2016/03/major-step-forward-addiction-medicine|website=National Institute on Drug Abuse|publisher=National Institutes of Health|accessdate=3 April 2016|date=31 March 2016|quote=Only about 10 percent of the 21 million Americans who meet the need for care for an alcohol or drug use disorder receive any form of treatment, and much of the treatment available does not meet standards for evidence-based care. There are many attitudinal and systemic reasons for this treatment gap, including stigma against treating people with addictions and institutional barriers to providing or funding addiction treatment. ... A major milestone was reached on March 14, 2016, when the American Board of Medical Specialties (ABMS) formally announced recognition of the field of Addiction Medicine as a medical subspecialty. ... In a statement issued to mark this milestone, ABAM President Robert J. Sokol summed up its significance: 'This landmark event, more than any other, recognizes addiction as a preventable and treatable disease, helping to shed the stigma that has long plagued it. It sends a strong message to the public that American medicine is committed to providing expert care for this disease and services designed to prevent the risky substance use that precedes it.'}}</ref> One-third of [[inpatient care|inpatient]] hospital costs and 20% of all deaths in the US every year are the result of untreated addictions and risky substance use.<ref name="ABAM" /><ref name="NIDA Addiction Medicine" /> In spite of the massive overall economic cost to society, which is greater than the cost of [[diabetes]] and all forms of [[cancer]] combined, most doctors in the US lack the training to effectively address a drug addiction.<ref name="ABAM" /><ref name="NIDA Addiction Medicine" />
| + | In 2019, opioid addiction was acknowledged as a national crisis in the United States. American drug companies were found to have flooded the country with prescription pain pills from 2006 through 2012, despite being aware that they were addictive and that they were fueling addiction and overdoses.<ref> Katelyn Newman, [https://www.usnews.com/news/healthiest-communities/articles/2019-07-17/drug-companies-pushed-76-billion-opioid-pills-across-us-from-2006-through-2012 Amid Opioid Crisis, Large Drug Companies Pushed 76 Billion Pain Pills Across U.S.] ''US News & World Report'', July 17, 2019. Retrieved August 31, 2022.</ref> |
| − | | |
| − | Another review listed estimates of lifetime prevalence rates for several behavioral addictions in the United States, including 1–2% for compulsive gambling, 5% for sexual addiction, 2.8% for food addiction, and 5–6% for compulsive shopping.<ref name="Natural and drug addictions" /> A systematic review indicated that the time-invariant prevalence rate for sexual addiction and related compulsive sexual behavior (e.g., compulsive masturbation with or without pornography, compulsive cybersex, etc.) within the United States ranges from 3–6% of the population.<ref name="Systematic review - yet another DSM fail">{{cite journal | vauthors = Karila L, Wéry A, Weinstein A, Cottencin O, Petit A, Reynaud M, Billieux J | title = Sexual addiction or hypersexual disorder: different terms for the same problem? A review of the literature | journal = Curr. Pharm. Des. | volume = 20 | issue = 25 | pages = 4012–20 | year = 2014 | pmid = 24001295 | doi = 10.2174/13816128113199990619| quote = Sexual addiction, which is also known as hypersexual disorder, has largely been ignored by psychiatrists, even though the condition causes serious psychosocial problems for many people. A lack of empirical evidence on sexual addiction is the result of the disease's complete absence from versions of the Diagnostic and Statistical Manual of Mental Disorders. ... Existing prevalence rates of sexual addiction-related disorders range from 3% to 6%. Sexual addiction/hypersexual disorder is used as an umbrella construct to encompass various types of problematic behaviors, including excessive masturbation, cybersex, pornography use, sexual behavior with consenting adults, telephone sex, strip club visitation, and other behaviors. The adverse consequences of sexual addiction are similar to the consequences of other addictive disorders. Addictive, somatic and psychiatric disorders coexist with sexual addiction. In recent years, research on sexual addiction has proliferated, and screening instruments have increasingly been developed to diagnose or quantify sexual addiction disorders. In our systematic review of the existing measures, 22 questionnaires were identified. As with other behavioral addictions, the appropriate treatment of sexual addiction should combine pharmacological and psychological approaches.}}</ref>
| |
| − | | |
| − | According to a 2017 poll conducted by the [[Pew Research Center]], almost half of US adults know a family member or close friend who has struggled with a drug addiction at some point in their life.<ref>{{cite web|title=Nearly half of Americans have a family member or close friend who's been addicted to drugs|url=http://www.pewresearch.org/fact-tank/2017/10/26/nearly-half-of-americans-have-a-family-member-or-close-friend-whos-been-addicted-to-drugs/|publisher=Pew Research Center|author=Gramlich J|date=26 October 2017|accessdate=14 January 2018}}</ref>
| |
| − | | |
| − | In 2019, opioid addiction was acknowledged as a national crisis in the United States.<ref>{{cite news |title=We were addicted to their pill, but they were addicted to the money |url=https://www.washingtonpost.com/video/national/we-were-addicted-to-their-pill-but-they-were-addicted-to-the-money/2019/07/21/7c006bc0-4e9b-46a5-bc80-c7e95e46d513_video.html?wpisrc=nl_headlines&wpmm=1 |accessdate=22 July 2019 |work=Washington Post |language=en}}</ref> A ''[[Washington Post]]'' article stated that "America’s largest drug companies flooded the country with pain pills from 2006 through 2012, even when it became apparent that they were fueling addiction and overdoses."
| |
| | | | |
| | ===South America=== | | ===South America=== |
| − | The realities of opioid use and abuse in Latin America may be deceptive if observations are limited to epidemiological findings. In the [[United Nations Office on Drugs and Crime]] report,<ref>[https://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf World Drug Report 2012] UN, 2012.</ref> although South America produced 3% of the world's morphine and heroin and 0.01% of its opium, prevalence of use is uneven. According to the Inter-American Commission on Drug Abuse Control, consumption of heroin is low in most Latin American countries, although Colombia is the area's largest opium producer. Mexico, because of its border with the United States, has the highest incidence of use.<ref>Pacurucu Castillo, José Marcelo, Adrián Hernández, Renato D. Alarcón (2019) [https://prcp.psychiatryonline.org/doi/10.1176/appi.prcp.20180009 "World Opioid and Substance Use Epidemic: A Latin American Perspective"] ''Psychiatryonline.org''</ref> | + | The realities of opioid use and abuse in Latin America may be deceptive if observations are limited to epidemiological findings. According to the Inter-American Commission on Drug Abuse Control, consumption of [[heroin]] is low in most Latin American countries, although [[Colombia]] is the area's largest [[opium]] producer. [[Mexico]], because of its border with the United States, has the highest incidence of use.<ref>Pacurucu Castillo, José Marcelo, Adrián Hernández, and Renato D. Alarcón, [https://prcp.psychiatryonline.org/doi/10.1176/appi.prcp.20180009 World Opioid and Substance Use Epidemic: A Latin American Perspective] ''Psychiatry Online.org'', January 24, 2019. Retrieved August 31, 2022.</ref> |
| − | | |
| − | ==Personality theories==
| |
| − | {{Main|Personality theories of addiction}}
| |
| − | [[Personality theories of addiction]] are [[psychology|psychological]] models that associate [[personality traits]] or modes of thinking (i.e., [[Affective science|affective states]]) with an individual's proclivity for developing an addiction. [[Data analysis]] demonstrates that there is a significant difference in the psychological profiles of drug users and non-users and the psychological predisposition to using different drugs may be different.<ref>{{cite book |last1= Fehrman|first1= Elaine|last2= Egan|first2=Vincent |last3= Gorban|first3= Alexander N. |last4= Levesley|first4= Jeremy |last5= Mirkes|first5= Evgeny M. |last6= Muhammad|first6=Awaz K. |title= Personality Traits and Drug Consumption. A Story Told by Data|url= |doi = 10.1007/978-3-030-10442-9|location= |publisher= Springer, Cham|page= |isbn=978-3-030-10441-2 |author-link= |arxiv= 2001.06520 |year= 2019}}</ref> Models of addiction risk that have been proposed in psychology literature include an [[affect dysregulation]] model of positive and negative [[psychological affect]]s, the [[reinforcement sensitivity theory]] model of [[impulsiveness]] and behavioral inhibition, and an impulsivity model of [[#Sensitization|reward sensitization]] and impulsiveness.<ref name="Cheetham_2010">{{cite journal |vauthors=Cheetham A, Allen NB, Yücel M, Lubman DI | title = The role of affective dysregulation in drug addiction | journal = Clin Psychol Rev | volume = 30 | issue = 6 | pages = 621–34 |date=August 2010| pmid = 20546986 | doi = 10.1016/j.cpr.2010.04.005 | url = }}</ref><ref name=Franken_2006>{{cite journal |vauthors=Franken IH, Muris P | title = BIS/BAS personality characteristics and college students' substance use|journal=Personality and Individual Differences | year = 2006 | volume = 40 | issue = 7 | pages = 1497–503 | doi = 10.1016/j.paid.2005.12.005 }}</ref><ref name="pmid18232522">{{cite journal |vauthors=Genovese JE, Wallace D | title = Reward sensitivity and substance abuse in middle school and high school students | journal = J Genet Psychol | volume = 168 | issue = 4 | pages = 465–69 |date=December 2007| pmid = 18232522 | doi = 10.3200/GNTP.168.4.465-469 }}</ref><ref name=Kimbrel_2007>{{cite journal |vauthors=Kimbrel NA, Nelson-Gray RO, Mitchell JT | title = Reinforcement sensitivity and maternal style as predictors of psychopathology | journal = Personality and Individual Differences |date=April 2007| volume = 42 | issue = 6 | pages = 1139–49 | doi = 10.1016/j.paid.2006.06.028 }}</ref><ref name="Dawe">{{cite journal |vauthors=Dawe S, Loxton NJ | title = The role of impulsivity in the development of substance use and eating disorders | journal = Neurosci Biobehav Rev | volume = 28 | issue = 3 | pages = 343–51 |date=May 2004| pmid = 15225976 | doi = 10.1016/j.neubiorev.2004.03.007 }}</ref>
| |
| | | | |
| | ==Notes== | | ==Notes== |
| − | {{reflist|group=note}}
| + | <references/> |
| − | | |
| − | ; Image legend
| |
| − | {{reflist|group=Color legend}}
| |
| | | | |
| | ==References== | | ==References== |
| − | | + | * American Psychiatric Association. ''Practice Guidelines for the Treatment of Psychiatric Disorders''. American Psychiatric Publishing, 2006. ISBN 978-0890423851 |
| − | | + | * American Psychiatric Association. ''Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5''. American Psychiatric Publishing, 2013. ISBN 978-0890425558 |
| − | * {{cite book |title=Unbroken Brain |author=Maia Szalavitz |year=2016 |isbn=978-1250055828 |publisher=St. Martin's Press |url-access=registration |url=https://archive.org/details/unbrokenbrainrev0000szal }} | + | * Fehrman, Elaine, Vincent Egan, Alexander N. Gorban, Jeremy Levesley, Evgeny M. Mirkes, and Awaz K. Muhammad. ''Personality Traits and Drug Consumption: A Story Told by Data''. Springer, 2019. ISBN 978-3030104412 |
| | + | * Ferri, Fred F. ''Ferri's Clinical Advisor 2020''. Elsevier, 2019. ISBN 978-0323672542 |
| | + | * Grant, Jon. ''Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions''. W. W. Norton & Company, 2008. ISBN 978-0393705218 |
| | + | * Nestler, Eric, Steven Hyman, and Robert Malenka. ''Molecular Neuropharmacology: A Foundation for Clinical Neuroscience''. McGraw-Hill, 2008. ISBN 978-0071481274 |
| | + | * Office of the Surgeon General. ''Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health''. CreateSpace, 2017. ISBN 978-1974580620 |
| | + | * Stein, Dan J., Eric Hollander, and Barbara O. Rothbaum (eds.). ''Textbook of Anxiety Disorders''. American Psychiatric Publishing, Inc., 2009. ISBN 1585622540 |
| | + | * Szalavitz, Maia. ''Unbroken Brain''. Picador, 2017. ISBN 978-1250116444 |
| | + | * United Nations Office on Drugs and Crime. ''International Narcotics Control Board Report: 2013''. United Nations, 2014. ISBN 978-9211482744 |
| | | | |
| | ==External links== | | ==External links== |
| − | | + | All links retrieved June 15, 2023. |
| − | * {{cite web |url=http://learn.genetics.utah.edu/content/addiction/ |title=The Science of Addiction: Genetics and the Brain |author=<!--Staff writer(s); no by-line.—> |date= |website=learn.genetics.utah.edu |publisher=Learn.Genetics – [[University of Utah]] |access-date= |quote=}} | + | * [https://learn.genetics.utah.edu/content/addiction/ The Science of Addiction: Genetics and the Brain] |
| − | * [http://www.tedmed.com/talks/show?id=309096 Why do our brains get addicted?] – a [[TEDMED]] 2014 talk by [[Nora Volkow]], the director of the [[National Institute on Drug Abuse]] at [[National Institutes of Health|NIH]]. | + | * Nora Volkow, [https://tedmed.com/talks/show?id=309096 Why do our brains get addicted?] ''TEDMED''. |
| − | * [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4811731/ Laterality of Brain Activation for Risk Factors of Addiction] | + | *Kyoto Encyclopedia of Genes and Genomes (KEGG) signal transduction pathways: |
| − | [[Kyoto Encyclopedia of Genes and Genomes]] (KEGG) signal transduction pathways:
| + | ** [https://www.genome.jp/kegg-bin/show_pathway?hsa05034+2354 KEGG – human alcohol addiction] |
| − | * [http://www.genome.jp/kegg-bin/show_pathway?hsa05034+2354 KEGG – human alcohol addiction] | + | ** [https://www.genome.jp/kegg-bin/show_pathway?hsa05031+2354 KEGG – human amphetamine addiction]https://www.genome.jp/kegg-bin/show_pathway?hsa05030+2354 KEGG – human cocaine addiction] |
| − | * [http://www.genome.jp/kegg-bin/show_pathway?hsa05031+2354 KEGG – human amphetamine addiction] | + | * [https://www.psy-ed.com/wpblog/child-gaming-addiction/ Is Your Child a Gaming Addict?] |
| − | * [http://www.genome.jp/kegg-bin/show_pathway?hsa05030+2354 KEGG – human cocaine addiction]
| + | * [https://safesoundtreatment.com/addictive-personality-facts-fiction/ Addictive Personality Traits: Facts and Fiction] |
| − | | + | * [https://enhancehealthgroup.com/outpatient-drug-rehab/ Outpatient Treatment for Addiction] |
| | + | * [https://nomatterwhatrecovery.com/addiction-prevention/ How To Help Prevent And Treat Substance Abuse] ''No Matter What Recovery'' |
| | + | * [https://www.inlanddetox.com/codependency-and-addiction/ Codependency & Addiction] ''Inland Detox'' |
| | | | |
| | [[Category:Psychology]] | | [[Category:Psychology]] |
| | [[Category:Health and disease]] | | [[Category:Health and disease]] |
| | | | |
| − | {{Credits|Addiction|956573121|Behavioral_addiction|959320931}} | + | {{Credits|Addiction|956573121|Behavioral_addiction|959320931|Substance_use_disorder|954950798}} |
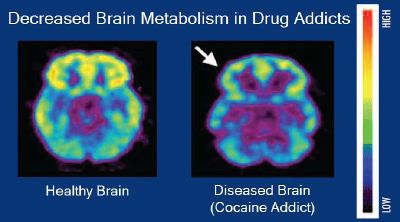
Brain positron emission tomography images that compare brain metabolism in a healthy individual and an individual with a
cocaine addiction
Addiction is a brain disorder characterized by compulsive engagement in rewarding stimuli despite adverse consequences. A number of psychosocial factors are involved, but it is a biological process—one that is induced by repeated exposure to an addictive stimulus—that is the core pathology that drives the development and maintenance of an addiction. Addictive stimuli are reinforcing and intrinsically rewarding.
Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences. Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs). Addiction has a massive overall economic cost to society, and, more importantly, is destructive to individuals, their families, and the social well-being of society as a whole.
Definition
The American Society of Addiction Medicine defines addiction as follows:
Addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences. People with addiction use substances or engage in behaviors that become compulsive and often continue despite harmful consequences.[1]
Did you know?
Addictions can be either to
substance abuse or behaviors that lead to a reward, such as
gambling, eating, or sexual activity
Addiction is a brain disorder characterized by compulsive engagement in rewarding stimuli despite adverse consequences.[2] The two properties that characterize all addictive stimuli are that they are reinforcing (in other words, they increase the likelihood that a person will seek repeated exposure to them) and intrinsically rewarding (meaning they are perceived as being inherently positive, desirable, and pleasurable).[3]
Classic hallmarks of addiction include impaired control over substances or behavior, preoccupation with substance or behavior, and continued use despite consequences. Habits and patterns associated with addiction are typically characterized by immediate gratification (short-term reward), coupled with delayed deleterious effects (long-term costs).[4]
Types of addiction
| Addiction and dependence glossary
|
* addiction – a biopsychosocial disorder characterized by compulsively seeking to achieve a desired effect, such as intoxication, despite harm and adverse consequences to self and others
- addictive behavior – a behavior that is both rewarding and reinforcing
- addictive drug – a drug that is both rewarding and reinforcing
- dependence – an adaptive state associated with a withdrawal syndrome upon cessation of repeated exposure to a stimulus (e.g., drug intake)
- drug sensitization or reverse tolerance – the escalating effect of a drug resulting from repeated administration at a given dose
- drug withdrawal – symptoms that occur upon cessation of repeated drug use
- physical dependence – dependence that involves persistent physical–somatic withdrawal symptoms (e.g., fatigue and delirium tremens)
- psychological dependence – dependence that involves emotional–motivational withdrawal symptoms (e.g., dysphoria and anhedonia)
- reinforcing stimuli – stimuli that increase the probability of repeating behaviors paired with them
- rewarding stimuli – stimuli that the brain interprets as intrinsically positive and desirable or as something to approach
- sensitization – an amplified response to a stimulus resulting from repeated exposure to it
- substance use disorder – a condition in which the use of substances leads to clinically and functionally significant impairment or distress
- tolerance – the diminishing effect of a drug resulting from repeated administration at a given dose
|
Addiction has traditionally been used in reference to substance abuse where there are obvious physical dependencies. However, the term has been expanded to include behaviors that may lead to a reward (such as gambling, eating, sexual activity, or even shopping).[5] A gene transcription factor known as ΔFosB has been identified as a necessary common factor involved in both behavioral and drug addictions, which are associated with the same set of neural adaptations in the reward system.[6][7]
Examples of drug and behavioral addictions include alcoholism, marijuana addiction, amphetamine addiction, cocaine addiction, nicotine addiction, opioid addiction, food addiction, video game addiction, gambling addiction, and sexual addiction. The only behavioral addiction recognized by the DSM-5 and the ICD-10 is gambling addiction. With the introduction of the ICD-11 gaming addiction was appended.[8]
The term addiction is misused frequently to refer to other compulsive behaviors or disorders, particularly dependence.[9] Substance dependence is an adaptive state that develops from repeated drug administration, and which results in withdrawal (a set of unpleasant physical symptoms that are opposite of the effects of the drug) upon cessation of use. Addiction is compulsive, out-of-control use of a substance or performance of a behavior despite negative consequences. Addiction can occur in the absence of dependence, and dependence can occur in the absence of addiction, although the two often occur together.
Biological mechanisms
ΔFosB, a gene transcription factor, has been identified as playing a critical role in the development of addictive states in both behavioral addictions and drug addictions.[6][10][7] Overexpression of ΔFosB in the nucleus accumbens is necessary and sufficient for many of the neural adaptations seen in drug addiction; it has been implicated in addictions to alcohol, cannabinoids, cocaine, nicotine, phenylcyclidine, and substituted amphetamines[6][11] as well as addictions to natural rewards such as sex, exercise, and food.[10][7]
In the nucleus accumbens, ΔFosB functions as a "sustained molecular switch" and "master control protein" in the development of an addiction. In other words, once "turned on" (sufficiently overexpressed) ΔFosB triggers a series of transcription events that ultimately produce an addictive state (compulsive reward-seeking involving a particular stimulus); this state is sustained for months after cessation of drug use due to the abnormal and exceptionally long half-life of ΔFosB isoforms.[12] ΔFosB expression in D1-type nucleus accumbens medium spiny neurons directly and positively regulates drug self-administration and reward sensitization through positive reinforcement while decreasing sensitivity to aversion.[13]
Besides increased ΔFosB expression in the nucleus accumbens, there are many other correlations in the neurobiology of behavioral addictions with drug addictions.
Behaviors like gambling have been linked to the brain's capacity to anticipate rewards. The reward system can be triggered by early detectors of the behavior, and trigger dopamine neurons to begin stimulating behaviors. But in some cases, it can lead to many issues due to error, or reward-prediction errors. These errors can act as teaching signals to create a complex behavior task over time.[14]
One of the most important discoveries of addictions has been the drug based reinforcement and, even more important, reward based learning processes. Several structures of the brain are important in the conditioning process of behavioral addiction; these subcortical structures form the brain regions known as the reward system. One of the major areas of study is the amygdala, a brain structure which involves emotional significance and associated learning. Research shows that dopaminergic projections from the ventral tegmental area facilitate a motivational or learned association to a specific behavior.[15] Dopamine neurons take a role in the learning and sustaining of many acquired behaviors. The most common mechanism of dopamine is to create addictive properties along with certain behaviors.[16]
There are three stages to the dopamine reward system: bursts of dopamine, triggering of behavior, and further impact to the behavior. Once electronically signaled, possibly through the behavior, dopamine neurons let out a ‘burst-fire’ of elements to stimulate areas along fast transmitting pathways. The behavior response then perpetuates the striated neurons to further send stimuli.[14] Once the behavior is triggered, it is difficult to work away from the dopamine reward system.
Substance use disorder
Substance use disorder (SUD), also known as a drug use disorder, is the persistent use of drugs (including alcohol) despite substantial harm and adverse consequences. Such addiction can be defined as "the compulsive seeking and taking of drugs despite horrendous consequences or loss of control over drug use."[13] Substance use disorders are characterized by an array of mental, physical, and behavioral symptoms that may cause problems related to loss of control, strain to one's interpersonal life, hazardous use, tolerance, and withdrawal.[17]
In the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), substance abuse and substance dependence were merged into the category of substance use disorders.ref name=DSM-5/> The severity of substance use disorders can vary widely; in the diagnosis of a SUD, the severity of an individual's SUD is qualified as mild, moderate, or severe on the basis of how many of the 11 diagnostic criteria are met.
Drug classes that are involved in SUD include: alcohol; caffeine; cannabis; hallucinogens (such as arylcyclohexylamines); other hallucinogens (such as LSD); inhalants; opioids; sedatives, hypnotics, or anxiolytics; stimulants; tobacco; and other or unknown substances.[18]
Addiction exacts an "astoundingly high financial and human toll" on individuals and society as a whole.[2] In the United States, the total economic cost to society is greater than that of all types of diabetes and all cancers combined:
Risky substance use and untreated addiction account for one-third of inpatient hospital costs and 20 percent of all deaths in the United States each year, and cause or contribute to more than 100 other conditions requiring medical care, as well as vehicular crashes, other fatal and non-fatal injuries, overdose deaths, suicides, homicides, domestic discord, the highest incarceration rate in the world and many other costly social consequences. The economic cost to society is greater than the cost of diabetes and all cancers combined.[19]
These costs arise from the direct adverse effects of drugs and associated healthcare costs, long-term complications (such as lung cancer from smoking tobacco products, liver cirrhosis and dementia from chronic alcohol consumption, and meth mouth from methamphetamine use), the loss of productivity and associated welfare costs, fatal and non-fatal accidents, suicides, homicides, and incarceration, among others.[20]
Diagnosis
Diagnosis of substance use disorder (SUD) usually involves an in-depth examination, typically by psychiatrist, psychologist, or drug and alcohol counselor.[21] The most commonly used guidelines are published in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).[17]
The 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) uses the term "substance use disorder" to refer to a spectrum of drug use-related disorders. The DSM-5 eliminates the terms "abuse" and "dependence" from diagnostic categories, instead using the specifiers of mild, moderate and severe to indicate the extent of disordered use. These specifiers are determined by the number of diagnostic criteria present in a given case. In the DSM-5, the term drug addiction is synonymous with severe substance use disorder.
There are 11 diagnostic criteria which can be broadly categorized into issues arising from substance use related to loss of control, strain to one's interpersonal life, hazardous use, and pharmacologic effects. DSM-5 guidelines for the diagnosis of a substance use disorder requires that the individual have significant impairment or distress from their pattern of drug use, and at least two of the symptoms listed below in a given year.[17]
- Using more of a substance than planned, or using a substance for a longer interval than desired
- Inability to cut down despite desire to do so
- Spending substantial amount of the day obtaining, using, or recovering from substance use
- Cravings or intense urges to use
- Repeated usage causes or contributes to an inability to meet important social, or professional obligations
- Persistent usage despite user's knowledge that it is causing frequent problems at work, school, or home
- Giving up or cutting back on important social, professional, or leisure activities because of use
- Using in physically hazardous situations, or usage causing physical or mental harm
- Persistent use despite the user's awareness that the substance is causing or at least worsening a physical or mental problem
- Tolerance: needing to use increasing amounts of a substance to obtain its desired effects
- Withdrawal: characteristic group of physical effects or symptoms that emerge as amount of substance in the body decreases
Tolerance is the process by which the body continually adapts to the substance and requires increasingly larger amounts to achieve the original effects. Physical dependence occurs when the body has adjusted by incorporating the substance into its "normal" functioning – attained homeostasis – and therefore physical withdrawal symptoms occur upon cessation of use. Symptoms of withdrawal generally include, but are not limited to, body aches, anxiety, irritability, intense cravings for the substance, nausea, hallucinations, headaches, cold sweats, tremors, and seizures.
There are additional qualifiers and exceptions outlined in the DSM. For instance, if an individual is taking opiates as prescribed, they may experience physiologic effects of tolerance and withdrawal, but this would not cause an individual to meet criteria for a SUD without additional symptoms also being present.[17]
Medical researchers who actively study addiction have criticized the DSM classification of addiction for being flawed and involving arbitrary diagnostic criteria.[2] Writing in 2013, Thomas Insel, the director of the United States National Institute of Mental Health discussed the invalidity of the DSM-5's classification of mental disorders:
While DSM has been described as a "Bible" for the field, it is, at best, a dictionary, creating a set of labels and defining each. The strength of each of the editions of DSM has been "reliability" – each edition has ensured that clinicians use the same terms in the same ways. The weakness is its lack of validity. Unlike our definitions of ischemic heart disease, lymphoma, or AIDS, the DSM diagnoses are based on a consensus about clusters of clinical symptoms, not any objective laboratory measure. In the rest of medicine, this would be equivalent to creating diagnostic systems based on the nature of chest pain or the quality of fever.[22]
Given that addiction manifests in structural changes to the brain, it is possible that non-invasive neuroimaging scans obtained via MRI could be used to help diagnose addiction in the future.[23] As a diagnostic biomarker, ΔFosB expression could be used to diagnose addiction, but this would require a brain biopsy and therefore is not used in clinical practice.
Treatment
Treatment for substance abuse disorder is not simple. Rather than a single treatment, a variety of different approaches are required for success:
In order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities.[3]
Detoxification
Depending on the severity of use, and the given substance, early treatment of acute withdrawal may include medical detoxification. Of note, acute withdrawal from heavy alcohol use must be done under medical supervision to prevent a potentially deadly withdrawal syndrome known as delirium tremens.
Therapy
Cognitive Behavioral Therapy (CBT) has been found moderately effective in treating addictions
Therapeutic treatments usually involve planning for specific ways to avoid the addictive stimulus, and therapeutic interventions intended to help a client learn healthier ways to find satisfaction. Therapists attempt to tailor intervention approaches to specific influences that affect addictive behavior, using therapeutic interviews in an effort to discover factors that led a person to embrace unhealthy, addictive sources of pleasure or relief from pain.
A meta-analytic review on the efficacy of various behavioral therapies for treating drug and behavioral addictions found that cognitive behavioral therapy (such as relapse prevention and contingency management), motivational interviewing, and a community reinforcement approach were effective interventions with moderate effect sizes.[24]
Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (such as marathon running), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and for psychostimulant addiction in particular.[10][25] Consistent aerobic exercise reduces drug addiction risk, decreases drug self-administration, reduces the likelihood of relapse, and induces opposite effects on striatal dopamine receptor D2 (DRD2) signaling (increased DRD2 density) to those induced by addictions to several drug classes (decreased DRD2 density). Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.[10][25]
Medication
Medication-assisted treatment (MAT) refers to the combination of behavioral interventions and medications to treat substance use disorders. Certain medications can be useful in treating severe substance use disorders. In the United States, several medications, such as disulfiram and methadone, are approved to treat alcohol and opioid use disorders.[26] There are no approved medications for cocaine, methamphetamine, or other substance use disorders.
Approved medications can be used as part of broader treatment plans to help a patient function comfortably without illicit opioids or alcohol.[27] Medications can be used in treatment to lessen withdrawal symptoms. Evidence has demonstrated the efficacy of MAT at reducing illicit drug use and overdose deaths, improving retention in treatment, and reducing HIV transmission.[28]
Alcohol addiction
Alcohol, like opioids, can induce a severe state of physical dependence and produce withdrawal symptoms such as delirium tremens. Because of this, treatment for alcohol addiction usually involves a combined approach dealing with dependence and addiction simultaneously. Benzodiazepines have the largest and the best evidence base in the treatment of alcohol withdrawal and are considered the gold standard of alcohol detoxification.[29]
Pharmacological treatments for alcohol addiction include naltrexone (opioid antagonist), disulfiram, acamprosate, and topiramate. Rather than substituting for alcohol, these drugs are intended to affect the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.[30]
Cannabinoid addiction
Cannabis is a widely used substance, and demand for effective treatment is increasing. However, abstinence rates following behavioral therapies have been modest, and there are no effective pharmacotherapies for the treatment of cannabis addiction.[31]
Nicotine addiction
Medication assisted treatment has been widely used is in the treatment of nicotine addiction. This usually involves nicotine replacement therapy, nicotinic receptor antagonists, or nicotinic receptor partial agonists. Drugs that act on nicotinic receptors and have been used for treating nicotine addiction include antagonists like bupropion and the partial agonist varenicline.[32]
Opioid addiction
Opioids cause physical dependence, and treatment typically addresses both dependence and addiction.
Physical dependence is treated using replacement drugs such as suboxone or subutex (both containing the active ingredients buprenorphine) and methadone.[33] Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a measure of control over both pain and cravings. Use of replacement drugs increases the addicted individual's ability to function normally and eliminates the negative consequences of obtaining controlled substances illicitly. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases.
In the United States, opiate replacement therapy is tightly regulated in methadone clinics and under the DATA 2000 legislation. In some countries, other opioid derivatives are used as substitute drugs for illegal street opiates, with different prescriptions being given depending on the needs of the individual patient.
Psychostimulant addiction
There is no effective pharmacotherapy for any form of psychostimulant addiction. Many drugs have been tested, but none have shown conclusive efficacy with tolerable side effects in humans.[3] Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved, and psychotherapy remains the mainstay of treatment.
Risk factors
There are many known risk factors associated with an increased chance of developing a substance use disorder (SUD). For example, children born to parents with SUDs have roughly a two-fold increased risk in developing an addiction compared to children born to parents without any SUDs. Other common risk factors are being male, being under 25, having other mental health problems, and lack of familial support and supervision.[34] Psychological risk factors include high impulsivity, sensation seeking, neuroticism, and openness to experience in combination with low conscientiousness.[35]
There are a number of genetic and environmental risk factors for developing an addiction, that vary across the population. Even in individuals with a relatively low genetic risk, exposure to sufficiently high doses of an addictive drug for a long period of time can result in an addiction.[13]
Genetic factors
It has long been established that genetic factors along with environmental (such as psychosocial) factors are significant contributors to addiction vulnerability.[13] Epidemiological studies estimate that genetic factors account for 40–60 percent of the risk factors for alcoholism.[36] Similar rates of heritability for other types of drug addiction have been indicated by other studies.[37]
Twin studies highlight the significant role genetics play in addiction. Rarely does only one twin have an addiction: In most cases where at least one twin suffered from an addiction, both did, and often to the same substance. Family studies reveal that if one family member has a history of addiction, the chances of a relative or close family developing an addiction to the same substance or a different addiction are much higher than one who has not been introduced to addiction at a young age. Such "cross addiction" occurs because all addictions work in the same part of the brain.[37]
Environmental factors
A number of different environmental factors have been implicated as risk factors for addiction, including various psychosocial stressors. However, an individual's exposure to an addictive drug is by far the most significant environmental risk factor for addiction.[13] The National Institute on Drug Abuse (NIDA) cites lack of parental supervision, the prevalence of peer substance use, drug availability, and poverty as risk factors for substance use among children and adolescents.[38]
Age
The earlier someone starts to use drugs, the higher the chance that they will grow to abuse or become dependent on them later on in life. Statistics have shown that those who start to drink alcohol at a younger age, especially prior to 12 years of age, are more likely to become dependent later on.[39]
Adolescence represents a period of unique vulnerability for developing an addiction. In adolescence, the incentive-rewards systems in the brain mature well before the cognitive control center. This consequentially grants the incentive-rewards systems a disproportionate amount of power in the behavioral decision-making process. Therefore, adolescents are increasingly likely to act on their impulses and engage in risky, potentially addicting behavior before considering the consequences.[40] Not only are adolescents more likely to initiate and maintain drug use, but once addicted they are more resistant to treatment and more liable to relapse.
Most individuals are exposed to and use addictive drugs for the first time during their teenage years. In the United States, for example, over 90 percent of those with an addiction began drinking, smoking, or using illicit drugs before the age of 18.[41]
Comorbid disorders
Individuals with comorbid (co-occurring) mental health disorders such as depression, anxiety, attention-deficit/hyperactivity disorder (ADHD), or post-traumatic stress disorder (PTSD) are more likely to develop substance use disorders.[42] The National Bureau of Economic Research reports a "definite connection between mental illness and the use of addictive substances," and "When other factors are held constant, mental illness does increase use of addictive goods — relative to use by the overall population — by 20 percent for alcohol, 27 percent for cocaine, and 86 percent for cigarettes."[43]
Epigenetic factors
Transgenerational epigenetic inheritance is the transmission of epigenetic markers from one generation to the next (parent–child transmission), affecting the traits and behavioral phenotypes of their offspring (for example, behavioral responses to environmental stimuli) without alteration of the primary structure of DNA (the sequence of nucleotides). In addiction, epigenetic mechanisms play a central role in the pathophysiology of the disease.[13] Some of the alterations to the epigenome which arise through chronic exposure to addictive stimuli during an addiction can be transmitted across generations, in turn affecting the behavior of one's children (such as the child's behavioral responses to addictive drugs and natural rewards).[44] However, the components that are responsible for the heritability of characteristics that make an individual more susceptible to drug addiction in humans remain largely unknown.
Behavioral addictions
Behavioral addiction is a form of addiction that involves a compulsion to engage in an inherently rewarding non-substance-related behavior – sometimes called a "natural reward"[6][10] – despite adverse consequences to the person's physical, mental, social, or financial well-being.[45][2]
Addiction to both drugs and behavioral rewards may arise from similar dysregulation of the mesolimbic dopamine system. Preclinical evidence has demonstrated that marked increases in the expression of ΔFosB through repetitive and excessive exposure to a natural reward induces the same behavioral effects and neuroplasticity as occurs in a drug addiction.[10]
Psychiatric and medical classifications
Behavioral addictions were introduced as a new diagnostic category in DSM-5, but only gambling addiction is included. Internet gaming addiction is included in the appendix as a condition for further study. Diagnostic models do not currently include the criteria necessary to identify behaviors as addictions in a clinical setting.
In September 2019, the American Society of Addiction Medicine (ASAM) issued a public statement defining all addiction in terms of brain changes:
Addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences. People with addiction use substances or engage in behaviors that become compulsive and often continue despite harmful consequences.[1]
The type of excessive behaviors identified as being addictive include gambling, food, chocolate, sexual intercourse, use of pornography, use of computers, playing video games, use of the internet and other digital media, exercise, and shopping.
Gambling provides a natural reward which is associated with compulsive behavior and for which clinical diagnostic manuals, namely the DSM-5, have identified diagnostic criteria for an addiction. In order for a person's gambling behavior to meet criteria of an addiction, it shows certain characteristics, such as mood modification, compulsivity, and withdrawal. There is evidence from functional neuroimaging that gambling activates the reward system and the mesolimbic pathway in particular.[46] Similarly, shopping and playing video games are associated with compulsive behaviors in humans and have also been shown to activate the mesolimbic pathway and other parts of the reward system.[10] Based upon this evidence, gambling addiction, video game addiction, and shopping addiction are classified accordingly.[10][46]
Reviews of both clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form of sexual intercourse – as an addiction. Moreover, reward cross-sensitization between amphetamine and sexual activity, meaning that exposure to one increases the desire for both, has been shown to occur preclinically and clinically as a dopamine dysregulation syndrome; ΔFosB expression is required for this cross-sensitization effect, which intensifies with the level of ΔFosB expression.[10]
Reviews of preclinical studies indicate that long-term frequent and excessive consumption of high fat or sugar foods can produce an addiction (food addiction).[10]
Excessive and compulsive Internet use has also been studied, revealing it to be a behavioral addiction with serious psychosocial consequences:
The growing number of researches on Internet addiction indicates that Internet addiction is a psychosocial disorder and its characteristics are as follows: tolerance, withdrawal symptoms, affective disorders, and problems in social relations. Internet usage creates psychological, social, school and/or work difficulties in a person's life. Eighteen percent of study participants were considered to be pathological Internet users, whose excessive use of the Internet was causing academic, social, and interpersonal problems. Excessive Internet use may create a heightened level of psychological arousal, resulting in little sleep, failure to eat for long periods, and limited physical activity, possibly leading to the user experiencing physical and mental health problems such as depression, OCD, low family relationships and anxiety.[47]
Studies on Internet addiction reveal the same fundamental brain changes seen in other addictions.[48][49]
Another growing area is social media addiction. Researchers found that not only is social media (particularly Facebook) itself potentially addictive, those who use it may also be at greater risk for substance abuse.[50]
Treatment
Behavioral addiction is a treatable condition.[51] Treatment options include psychotherapy and psychopharmacotherapy (medications) or a combination of both. Cognitive behavioral therapy (CBT) is the most common form of psychotherapy used in treating behavioral addictions; it focuses on identifying patterns that trigger compulsive behavior and making lifestyle changes to promote healthier behaviors. While CBT does not cure behavioral addiction, it does help with coping with the condition in a healthy way.
Currently, there are no medications approved for treatment of behavioral addictions in general, but some medications used for treatment of drug addiction may also be beneficial with specific behavioral addictions.[46] Any unrelated psychiatric disorders should be kept under control, and differentiated from the contributing factors that cause the addiction.
Epidemiology
Due to cultural variations, the proportion of individuals who develop a drug or behavioral addiction within a specified time period (the prevalence) varies over time, by country, and across national population demographics (age group, socioeconomic status, and so forth).[44]
Asia
The prevalence of alcohol dependence is not as high as is seen in other regions. In Asia, not only socioeconomic factors but also biological factors influence drinking behavior.[52]
Europe and Oceania
A 2017 report noted that Eastern Europe had the highest mortality rates for alcohol and illicit drugs, while Oceania had the highest tobacco mortality rates.[53]
United States
Addiction is widespread in the United States. According to a 2017 poll conducted by the Pew Research Center, almost half of US adults know a family member or close friend who has struggled with a drug addiction at some point in their life.[54]
In spite of the massive overall economic cost to society, which is greater than the cost of diabetes and all forms of cancer combined, most doctors in the US lack the training to effectively address a drug addiction.[19] In 2016, it was reported that only about ten percent, or a little over 2 million, receive any form of treatments, and those that do generally do not receive evidence-based care. A major milestone was reached on March 14, 2016, when the American Board of Medical Specialties (ABMS) formally announced recognition of the field of Addiction Medicine as a medical subspecialty:
This landmark event, more than any other, recognizes addiction as a preventable and treatable disease, helping to shed the stigma that has long plagued it. It sends a strong message to the public that American medicine is committed to providing expert care for this disease and services designed to prevent the risky substance use that precedes it.[55]
In 2019, opioid addiction was acknowledged as a national crisis in the United States. American drug companies were found to have flooded the country with prescription pain pills from 2006 through 2012, despite being aware that they were addictive and that they were fueling addiction and overdoses.[56]
South America
The realities of opioid use and abuse in Latin America may be deceptive if observations are limited to epidemiological findings. According to the Inter-American Commission on Drug Abuse Control, consumption of heroin is low in most Latin American countries, although Colombia is the area's largest opium producer. Mexico, because of its border with the United States, has the highest incidence of use.[57]
Notes
- ↑ 1.0 1.1 What is the definition of addiction? American Society of Addiction Medicine. Retrieved August 31, 2022.
- ↑ 2.0 2.1 2.2 2.3 Eric Nestler, Steven Hyman, and Robert Malenka, Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (McGraw-Hill, 2008, ISBN 978-0071481274).
- ↑ 3.0 3.1 3.2 Sara B. Taylor, Candace R. Lewis, and M. Foster Olive, The Neurocircuitry of Illicit Psychostimulant Addiction: Acute and Chronic Effects in Humans Subst Abuse Rehabil 8(4) (2013):29-43. Retrieved August 31, 2022.
- ↑ G.A. Marlatt, J.S. Baer, D.M. Donovan, and D.R. Kivlahan, Addictive Behaviors: Etiology and Treatment Annual Review of Psychology 39 (1988): 223–252. Retrieved August 31, 2022.
- ↑ Constance Holden, 'Behavioral' Addictions: Do They Exist? Science 294(5544) (2001): 980–982. Retrieved August 31, 2022.
- ↑ 6.0 6.1 6.2 6.3 Alfred J. Robison and Eric J. Nestler, Transcriptional and Epigenetic Mechanisms of Addiction Nature Reviews Neuroscience 12(11) (2011):623-637. Retrieved August 31, 2022.
- ↑ 7.0 7.1 7.2 Kenneth Blum et al., Sex, Drugs, and Rock ‘N’ Roll: Hypothesizing Common Mesolimbic Activation as a Function of Reward Gene Polymorphisms Psychoactive Drugs 44(1) (2012):38–55. Retrieved August 31, 2022.
- ↑ Meredith E. Gansner, Gaming Addiction in ICD-11: Issues and Implications Psychiatric Times, September 12, 2019. Retrieved August 31, 2022.
- ↑ Substance-Related and Addictive Disorders Mental Health Gateway. Retrieved August 31, 2022.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 10.9 Christopher M. Olsen, Natural Rewards, Neuroplasticity, and Non-Drug Addictions Neuropharmacology 61(7) (2011): 1109–1122. Retrieved August 31, 2022.
- ↑ Steven E. Hyman, Robert C. Malenka, and Eric J. Nestler, Neural mechanisms of addiction: the role of reward-related learning and memory Annual Review of Neuroscience 29 (2006):565–598. Retrieved August 31, 2022.
- ↑ E.J. Nestler, M. Barrot, and D.W. Self, DeltaFosB: A Sustained Molecular Switch for Addiction Proc Natl Acad Sci U S A 98(20) (2001):11042-22046. Retrieved August 31, 2022.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Eric J. Nestler, Cellular basis of memory for addiction Dialogues Clin Neurosci 15(4) (2013): 431–443. Retrieved August 31, 2022.
- ↑ 14.0 14.1 Gaetano Di Chiara and Valentina Bassareo, Reward System and Addiction: What Dopamine Does and Doesn't Do Current Opinion in Pharmacology 7(1) (2007):69–76. Retrieved August 31, 2022.
- ↑ Judson A. Brewer and Marc N. Potenza, The neurobiology and genetics of impulse control disorders: Relationships to drug addictions Biochemical Pharmacology 75(1) (2008):63–75. Retrieved August 31, 2022.
- ↑ Jean-Antoine Girault and Paul Greengard, The Neurobiology of Dopamine Signaling Archives of Neurology 61(5) (2004):641–644. Retrieved August 31, 2022.
- ↑ 17.0 17.1 17.2 17.3 American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5 (American Psychiatric Publishing, 2013, ISBN 978-0890425558).
- ↑ Substance Abuse and Mental Health Services Administration, Substance Use Disorders Impact of the DSM-IV to DSM-5 Changes on the National Survey on Drug Use and Health, 2016. Retrieved August 31, 2022.
- ↑ 19.0 19.1 Dennis Tartaglia, ABMS Officially Recognizes Addiction Medicine as a Subspecialty American Board of Addiction Medicine, March 14, 2016. Retrieved August 31, 2022.
- ↑ United Nations Office on Drugs and Crime, International Narcotics Control Board Report: 2013 (United Nations, 2014, ISBN 978-9211482744).
- ↑ Drug addiction (substance use disorder) Mayo Clinic. Retrieved August 31, 2022.
- ↑ John Horgan, Psychiatry in Crisis! Mental Health Director Rejects Psychiatric "Bible" and Replaces With Nothing Scientific American, May 4, 2013. Retrieved August 31, 2022.
- ↑ William H. Hampton, Italia M. Hanik, and Ingrid R. Olson, Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research Drug and Alcohol Dependence 197(4) (2019):288–298. Retrieved August 31, 2022.
- ↑ M. Walter, et al., Psychosocial Treatment of Addictive Disorders – An Overview of Psychotherapeutic Options and their Efficacy Fortschr Neurol Psychiatr 83(4) (2015):201–210. Retrieved August 31, 2022.
- ↑ 25.0 25.1 Wendy J Lynch, Alexis B Peterson, Victoria Sanchez, Jean Abel, and Mark A. Smith, Exercise as a Novel Treatment for Drug Addiction: A Neurobiological and Stage-Dependent Hypothesis Neurosci Biobehav Rev 37(8) (2013):1622–1644. Retrieved August 31, 2022.
- ↑ American Psychiatric Association, Practice Guidelines for the Treatment of Psychiatric Disorders (American Psychiatric Publishing, 2006, ISBN 978-0890423851).
- ↑ Antoine B. Douaihy, Thomas M. Kelly, and Carl Sullivan, Medications for Substance Use Disorders Soc Work Public Health 28(0) (2013): 264–278. Retrieved August 31, 2022.
- ↑ Office of the Surgeon General, Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health (CreateSpace, 2017, ISBN 978-1974580620).
- ↑ Ankur Sachdeva, Mona Choudhary, and Mina Chandra, Alcohol Withdrawal Syndrome: Benzodiazepines and Beyond Journal of Clinical and Diagnostic Research 9(9) (2015):VE01–VE07. Retrieved August 31, 2022.
- ↑ Bradford T. Winslow, Mary Onysko, and Melanie Hebert, Medications for Alcohol Use Disorder American Family Physician 93(6) (2016):457-465. Retrieved August 31, 2022.
- ↑ Mehmet Sofuoglu, Dawn E. Sugarman, and Kathleen M. Carroll, Cognitive Function as an Emerging Treatment Target for Marijuana Addiction Exp Clin Psychopharmacol. 18(2) (2010): 109–119. Retrieved August 31, 2022.
- ↑ Peter A. Crooks, Michael T. Bardo, and Linda P. Dwoskin, Nicotinic Receptor Antagonists as Treatments for Nicotine Abuse Adv Pharmacol 69 (2014):513-551. Retrieved August 31, 2022.
- ↑ M. Connock et al., Methadone and Buprenorphine for the Management of Opioid Dependence: A Systematic Review and Economic Evaluation Health Technol Assess 11(9) (2007):1-171. Retrieved August 31, 2022.
- ↑ Fred F. Ferri, Ferri's Clinical Advisor 2020 (Elsevier, 2019, ISBN 978-0323672542).
- ↑ Elaine Fehrman, Vincent Egan, Alexander N. Gorban, Jeremy Levesley, Evgeny M. Mirkes, and Awaz K. Muhammad, Personality Traits and Drug Consumption: A Story Told by Data (Springer, 2019, ISBN 978-3030104412).
- ↑ R.D. Mayfield, R.A. Harris, and M.A. Schuckit, Genetic factors influencing alcohol dependence Br J Pharmacol 154(2) (2008): 275–287. Retrieved August 31, 2022.
- ↑ 37.0 37.1 Steven M. Melemis, The Genetics of Drug and Alcohol Addiction Addictions and Recovery. Retrieved August 31, 2022.
- ↑ What are risk factors and protective factors? National Institute on Drug Abuse, October 2011.
- ↑ Age and Substance Abuse Alcohol Rehab. Retrieved August 31, 2022.
- ↑ Christopher J. Hammond, Linda C. Mayes, and Marc N. Potenza, Neurobiology of Adolescent Substance Use and Addictive Behaviors: Prevention and Treatment Implications Adolesc Med State Art Rev 25(1) (2014): 15–32. Retrieved August 31, 2022.
- ↑ Statistics on Addiction in America Addiction Center. Retrieved August 31, 2022.
- ↑ Drug addiction (substance use disorder): Risk factors Mayo Clinic. Retrieved August 31, 2022.
- ↑ Marie Bussing-Birks, Mental Illness and Substance Abuse National Bureau of Economic Research. Retrieved August 31, 2022.
- ↑ 44.0 44.1 F.M. Vassoler and G. Sadri-Vakili, Mechanisms of transgenerational inheritance of addictive-like behaviors Neuroscience 264 (2014): 198–206. Retrieved August 31, 2022.
- ↑ Dan J. Stein, Eric Hollander, and Barbara O. Rothbaum (eds.), Textbook of Anxiety Disorders (American Psychiatric Publishing, Inc., 2009, ISBN 1585622540).
- ↑ 46.0 46.1 46.2 Jon E. Grant, Marc N. Potenza, Aviv Weinstein, and David A. Gorelick, Introduction to Behavioral Addictions Am J Drug Alcohol Abuse 36(5) (2010):233-241. Retrieved August 31, 2022.
- ↑ Seyyed Salman Alavi, Mohammad Reza Maracy, Fereshte Jannatifard, and Mehdi Eslami, The effect of psychiatric symptoms on the internet addiction disorder in Isfahan's University students J Res Med Sci. 16(6) (2011):793–800. Retrieved August 31, 2022.
- ↑ Fuchun Lin, Yan Zhou, Yasong Du, Lindi Qin, Zhimin Zhao, Jianrong Xu, and Hao Lei, Abnormal White Matter Integrity in Adolescents with Internet Addiction Disorder: A Tract-Based Spatial Statistics Study PLoS One 7(1) (2012). Retrieved August 31, 2022.
- ↑ Sang Hee Kim, Sang-Hyun Baik, Chang Soo Park, Su Jin Kim, Sung Won Choi, and Sang Eun Kim, Reduced Striatal Dopamine D2 Receptors in People With Internet Addiction Neuroreport 22(8) (2011):407-411. Retrieved August 31, 2022.
- ↑ Craving Facebook? UAlbany Study Finds Social Media to be Potentially Addictive, Associated with Substance Abuse News Center, University at Albany, State University of New York, December 9, 2014. Retrieved August 31, 2022.
- ↑ Jon Grant, Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions (W. W. Norton & Company, 2008, ISBN 978-0393705218).
- ↑ Chiao-Chicy Chen and Shih-Jiun Yin, Alcohol abuse and related factors in Asia Int. Rev. Psychiatry 20(5) (2008):425-433. Retrieved August 31, 2022.
- ↑ Amy Peacock et al., Global statistics on alcohol, tobacco and illicit drug use: 2017 status report Addiction 113(10) (2018):1905-1926. Retrieved August 31, 2022.
- ↑ John Gramlich, Nearly half of Americans have a family member or close friend who's been addicted to drugs Pew Research Center, October 26, 2017. Retrieved August 31, 2022.
- ↑ Nora Volkow, A Major Step Forward for Addiction Medicine National Institute on Drug Abuse, March 31, 2016. Retrieved August 31, 2022.
- ↑ Katelyn Newman, Amid Opioid Crisis, Large Drug Companies Pushed 76 Billion Pain Pills Across U.S. US News & World Report, July 17, 2019. Retrieved August 31, 2022.
- ↑ Pacurucu Castillo, José Marcelo, Adrián Hernández, and Renato D. Alarcón, World Opioid and Substance Use Epidemic: A Latin American Perspective Psychiatry Online.org, January 24, 2019. Retrieved August 31, 2022.
References
ISBN links support NWE through referral fees
- American Psychiatric Association. Practice Guidelines for the Treatment of Psychiatric Disorders. American Psychiatric Publishing, 2006. ISBN 978-0890423851
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. American Psychiatric Publishing, 2013. ISBN 978-0890425558
- Fehrman, Elaine, Vincent Egan, Alexander N. Gorban, Jeremy Levesley, Evgeny M. Mirkes, and Awaz K. Muhammad. Personality Traits and Drug Consumption: A Story Told by Data. Springer, 2019. ISBN 978-3030104412
- Ferri, Fred F. Ferri's Clinical Advisor 2020. Elsevier, 2019. ISBN 978-0323672542
- Grant, Jon. Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions. W. W. Norton & Company, 2008. ISBN 978-0393705218
- Nestler, Eric, Steven Hyman, and Robert Malenka. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. McGraw-Hill, 2008. ISBN 978-0071481274
- Office of the Surgeon General. Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health. CreateSpace, 2017. ISBN 978-1974580620
- Stein, Dan J., Eric Hollander, and Barbara O. Rothbaum (eds.). Textbook of Anxiety Disorders. American Psychiatric Publishing, Inc., 2009. ISBN 1585622540
- Szalavitz, Maia. Unbroken Brain. Picador, 2017. ISBN 978-1250116444
- United Nations Office on Drugs and Crime. International Narcotics Control Board Report: 2013. United Nations, 2014. ISBN 978-9211482744
External links
All links retrieved June 15, 2023.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article
in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.

