Difference between revisions of "Deoxyribose" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 1: | Line 1: | ||
{{Claimed}}{{Contracted}} | {{Claimed}}{{Contracted}} | ||
[[Image:Deoxyribose.svg|thumb|right|200px|Deoxyribose]] | [[Image:Deoxyribose.svg|thumb|right|200px|Deoxyribose]] | ||
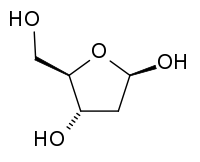
| − | '''Deoxyribose''', also known as '''<small>D</small>-Deoxyribose''' and '''2-deoxyribose''', is an | + | '''Deoxyribose''', also known as '''<small>D</small>-Deoxyribose''' and '''2-deoxyribose''', is an aldopentose—a ([[carbohydrate#Monosaccarides|monosaccharide]] containing five [[carbon]] [[atom]]s, and including an [[aldehyde]] functional group—that is the [[carbohydrate|sugar]] component of the nucleic acid [[DNA]] (deoxyribonucleic acid). It is derived from the [[ribose]], a pentose [[sugar]] (monosaccharide with five carbon atoms). Deoxyribose has the chemical formula {{carbon}}<sub>5</sub>{{hydrogen}}<sub>10</sub>{{oxygen}}<sub>4</sub>; |
| + | |||
| + | pentose sugar (monosaccharide with five carbon atoms) that is an important component | ||
| + | |||
| + | Note: [[Ribonucleic acid]] (RNA) is a nucleic acid based on the sugar ribose. [[Deoxyribonucleic acid]] (DNA) is a nucleic acid based on the closely related sugar deoxyribose. The bases in these nucleic acids ([[adenine]], [[uracil]], [[guanine]], and [[cytosine]] in RNA, and [[thymine]] instead of uracil in DNA) represents the genetic information in living cells. As a component of RNA, which is used for genetic transcription, ribose is critical to living creatures. | ||
| + | |||
| + | Note: A nucleic acid constituent (see illustration) of all animal, microbial, and plant cells; also known as 2-D-deoxyribose. Deoxyribose is enzymically formed in living cells by reduction of ribonucleoside di- or triphosphate. The four deoxyribose nucleotides, containing adenine, guanine, cytosine, and thymine, are the major constituents of the deoxyribonucleic acids (DNA), which control the hereditary characteristics of every living organism | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ==Structure== | ||
| + | [[Image:ribose.png|right|thumb|200px|Ribose in acyclic form]] | ||
| + | [[Image:Ribofuranose-2D-skeletal.png|right|thumb|200px|A conventional skeletal formula]] | ||
| + | |||
| + | Like ribose, deoxyribose is an Ribose is an aldopentose, which means a pentose [[carbohydrate|sugar]] with an aldehyde functional group in position 1. An aldehyde group consists of a carbon atom that is bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-). | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Note: [[Deoxyribose]], also known as 2-deoxyribose, is also an aldopentose. It is derived from ribose by the replacement of the hydroxyl group at the 2 position (the carbon furthest from the attached carbon) with [[hydrogen]], leading to the net loss of an [[oxygen]] atom. DNA has the chemical formula {{carbon}}<sub>5</sub>{{hydrogen}}<sub>10</sub>{{oxygen}}<sub>4</sub>. | ||
| + | |||
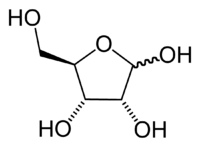
| + | Ribose has the chemical formula {{carbon}}<sub>5</sub>{{hydrogen}}<sub>10</sub>{{oxygen}}<sub>5</sub>. | ||
[[Ribose]] forms a five-member ring composed of four carbon atoms and one oxygen. [[Hydroxyl]] groups are attached to three of the carbons. The other carbon and a hydroxyl group are attached to one of the carbon atoms adjacent to the oxygen. In deoxyribose, the carbon furthest from the attached carbon is stripped of the oxygen atom in what would be a [[hydroxyl group]] in ribose. Due to the common C3 and C4 [[stereochemistry]] of D-ribose and D-arabinose, D-2-deoxyribose is also D-2-deoxyarabinose. | [[Ribose]] forms a five-member ring composed of four carbon atoms and one oxygen. [[Hydroxyl]] groups are attached to three of the carbons. The other carbon and a hydroxyl group are attached to one of the carbon atoms adjacent to the oxygen. In deoxyribose, the carbon furthest from the attached carbon is stripped of the oxygen atom in what would be a [[hydroxyl group]] in ribose. Due to the common C3 and C4 [[stereochemistry]] of D-ribose and D-arabinose, D-2-deoxyribose is also D-2-deoxyarabinose. | ||
'''Deoxyribofuranose''' is an alternative name for the ring structure of deoxyribose. This alternative name merely refers to the fact that deoxyribose has a five membered ring consisting of four carbons and an oxygen and is more a structural description than a name. | '''Deoxyribofuranose''' is an alternative name for the ring structure of deoxyribose. This alternative name merely refers to the fact that deoxyribose has a five membered ring consisting of four carbons and an oxygen and is more a structural description than a name. | ||
| + | |||
| + | |||
| + | Note: It is derived from the pentose sugar, ribose, by the replacement of the hydroxyl group at the 2 position with hydrogen, leading to the net loss of an oxygen atom. The chemical formula is C5H10O4 by the replacement of the [[hydroxyl group]] at the 2 position with [[hydrogen]], leading to the net loss of an [[oxygen]] atom, and | ||
| + | |||
| + | |||
| + | |||
| + | Note: Ribose forms a five-member ring composed of four carbon atoms and one oxygen. Hydroxyl (-OH) groups are attached to three of the carbons. The fourth carbon in the ring (one of the carbon atoms adjacent to the oxygen) has attached to it the fifth carbon atom and a hydroxyl group. | ||
| + | |||
| + | |||
| + | it was discovered in [[1929]] by [[Phoebus Levene]]. Ribose was discovered in 1909 by [[Phoebus Levene]]*, who also discovered DNA (1929) and found that DNA contained adenine, guanine, thymine, cytosine, deoxyribose, and a phosphate group. | ||
| + | |||
| + | |||
== Biological importance of deoxyribose == | == Biological importance of deoxyribose == | ||
| Line 22: | Line 56: | ||
In contrast, very similar molecules, containing ribose instead of deoxyribose, and known generically as [[RNA]], are known to form only relatively ''short'' double-helical complementary base paired structures. These are well known, for instance, in [[ribosome | ribosomal]] RNA molecules and in transfer RNA ([[tRNA]]), where so-called ''hairpin'' structures form from [[palindrome | palindromic]] sequences within one molecule. | In contrast, very similar molecules, containing ribose instead of deoxyribose, and known generically as [[RNA]], are known to form only relatively ''short'' double-helical complementary base paired structures. These are well known, for instance, in [[ribosome | ribosomal]] RNA molecules and in transfer RNA ([[tRNA]]), where so-called ''hairpin'' structures form from [[palindrome | palindromic]] sequences within one molecule. | ||
| + | |||
| + | |||
| + | Note: There are important diphosphate dimers called [[coenzyme]]s that [[purine]]s and [[pyrimidine]]s form with ribose. When these purine and pyrimidine derivatives are coupled to a ribose sugar, they are called [[nucleoside]]s. In these compounds, the convention is to put a ′(pronounced "prime") after the carbon numbers of the sugar, so that in nucleoside derivatives a name might include, for instance, the term "5′-monophosphate", meaning that the phosphate group is attached to the fifth carbon of the sugar, and not to the base. The bases are attached to the 1′ribose carbon in the common nucleosides. | ||
| + | |||
| + | Phosphorylated nucleosides are called [[nucleotide]]s. | ||
| + | |||
| + | The most common bases in nucleotides are: | ||
| + | *The purines [[adenine]] and [[guanine]]; | ||
| + | *The pyrimidines [[cytosine]], [[thymine]], and [[uracil]]; and | ||
| + | *The pyridine nicotinamide. | ||
| + | |||
| + | The sugar component is either ribose or deoxyribose. (“Deoxy” simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.) Depending on their base sugar, nucleotides are therefore known as “deoxyribonucleotides” or “ribonucleotides.” The nucleic acid [[DNA]] is built of nucleotides with a deoxyribose sugar, whereas [[RNA]] contains nucleotides composed of ribose sugars. | ||
| + | |||
== See also == | == See also == | ||
Revision as of 01:53, 12 March 2007
Deoxyribose, also known as D-Deoxyribose and 2-deoxyribose, is an aldopentose—a (monosaccharide containing five carbon atoms, and including an aldehyde functional group—that is the sugar component of the nucleic acid DNA (deoxyribonucleic acid). It is derived from the ribose, a pentose sugar (monosaccharide with five carbon atoms). Deoxyribose has the chemical formula C5H10O4;
pentose sugar (monosaccharide with five carbon atoms) that is an important component
Note: Ribonucleic acid (RNA) is a nucleic acid based on the sugar ribose. Deoxyribonucleic acid (DNA) is a nucleic acid based on the closely related sugar deoxyribose. The bases in these nucleic acids (adenine, uracil, guanine, and cytosine in RNA, and thymine instead of uracil in DNA) represents the genetic information in living cells. As a component of RNA, which is used for genetic transcription, ribose is critical to living creatures.
Note: A nucleic acid constituent (see illustration) of all animal, microbial, and plant cells; also known as 2-D-deoxyribose. Deoxyribose is enzymically formed in living cells by reduction of ribonucleoside di- or triphosphate. The four deoxyribose nucleotides, containing adenine, guanine, cytosine, and thymine, are the major constituents of the deoxyribonucleic acids (DNA), which control the hereditary characteristics of every living organism
Structure
Like ribose, deoxyribose is an Ribose is an aldopentose, which means a pentose sugar with an aldehyde functional group in position 1. An aldehyde group consists of a carbon atom that is bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-).
Note: Deoxyribose, also known as 2-deoxyribose, is also an aldopentose. It is derived from ribose by the replacement of the hydroxyl group at the 2 position (the carbon furthest from the attached carbon) with hydrogen, leading to the net loss of an oxygen atom. DNA has the chemical formula C5H10O4.
Ribose has the chemical formula C5H10O5.
Ribose forms a five-member ring composed of four carbon atoms and one oxygen. Hydroxyl groups are attached to three of the carbons. The other carbon and a hydroxyl group are attached to one of the carbon atoms adjacent to the oxygen. In deoxyribose, the carbon furthest from the attached carbon is stripped of the oxygen atom in what would be a hydroxyl group in ribose. Due to the common C3 and C4 stereochemistry of D-ribose and D-arabinose, D-2-deoxyribose is also D-2-deoxyarabinose.
Deoxyribofuranose is an alternative name for the ring structure of deoxyribose. This alternative name merely refers to the fact that deoxyribose has a five membered ring consisting of four carbons and an oxygen and is more a structural description than a name.
Note: It is derived from the pentose sugar, ribose, by the replacement of the hydroxyl group at the 2 position with hydrogen, leading to the net loss of an oxygen atom. The chemical formula is C5H10O4 by the replacement of the hydroxyl group at the 2 position with hydrogen, leading to the net loss of an oxygen atom, and
Note: Ribose forms a five-member ring composed of four carbon atoms and one oxygen. Hydroxyl (-OH) groups are attached to three of the carbons. The fourth carbon in the ring (one of the carbon atoms adjacent to the oxygen) has attached to it the fifth carbon atom and a hydroxyl group.
it was discovered in 1929 by Phoebus Levene. Ribose was discovered in 1909 by Phoebus Levene, who also discovered DNA (1929) and found that DNA contained adenine, guanine, thymine, cytosine, deoxyribose, and a phosphate group.
Biological importance of deoxyribose
Ribose and 2-deoxyribose derivatives have an important role in biology. Among the most important derivatives are those with phosphate groups attached at the 5 position. Mono-, di-, and triphosphate forms are important, as well as 3-5 cyclic monophosphates. There are also important diphosphate dimers called coenzymes that purines and pyrimidines form an important class of compounds with ribose and deoxyribose. When these purine and pyrimidine derivatives are coupled to a ribose sugar, they are called nucleosides. In these compounds, the convention is to put a ′ (pronounced "prime") after the carbon numbers of the sugar, so that in nucleoside derivatives a name might include, for instance, the term "5′-monophosphate", meaning that the phosphate group is attached to the fifth carbon of the sugar, and not to the base. The bases are attached to the 1′ ribose carbon in the common nucleosides. Phosphorylated nucleosides are called nucleotides.
One of the common bases is adenine (a purine derivative); coupled to ribose it is called adenosine; coupled to deoxyribose it is called deoxyadenosine. The 5′-triphosphate derivative of adenosine, commonly called ATP, for adenosine triphosphate, is an important energy transport molecule in cells.
See Nucleic acid nomenclature for a diagram showing the numbered positions in a 5′-monophosphate nucleotide.
2-Deoxyribose and ribose nucleotides are often found in unbranched 5′-3′ polymers. In these structures, the 3′carbon of one monomer unit is linked to a phosphate that is attached to the 5′carbon of the next unit, and so on. These polymer chains often contain many millions of monomer units. Since long polymers have physical properties distinctly different from those of small molecules, they are called macromolecules. The sugar-phosphate-sugar chain is called the backbone of the polymer. One end of the backbone has a free 5′phosphate, and the other end has a free 3′OH group. The backbone structure is independent of which particular bases are attached to the individual sugars.
Genetic material in earthly life often contains poly 5′-3′, 2′-deoxyribose nucleotides, in structures called chromosomes, where each monomer is one of the nucleotides deoxy- adenine, thymine, guanine or cytosine. This material is commonly called deoxyribonucleic acid, or simply DNA for short.
DNA in chromosomes forms very long helical structures containing two molecules with the backbones running in opposite directions on the outside of the helix and held together by hydrogen bonds between complementary nucleotide bases lying between the helical backbones. The lack of the 2′ hydroxyl group in DNA appears to allow the backbone the flexibility to assume the full conformation of the long double-helix, which involves not only the basic helix, but additional coiling necessary to fit these very long molecules into the very small volume of a cell nucleus.
In contrast, very similar molecules, containing ribose instead of deoxyribose, and known generically as RNA, are known to form only relatively short double-helical complementary base paired structures. These are well known, for instance, in ribosomal RNA molecules and in transfer RNA (tRNA), where so-called hairpin structures form from palindromic sequences within one molecule.
Note: There are important diphosphate dimers called coenzymes that purines and pyrimidines form with ribose. When these purine and pyrimidine derivatives are coupled to a ribose sugar, they are called nucleosides. In these compounds, the convention is to put a ′(pronounced "prime") after the carbon numbers of the sugar, so that in nucleoside derivatives a name might include, for instance, the term "5′-monophosphate", meaning that the phosphate group is attached to the fifth carbon of the sugar, and not to the base. The bases are attached to the 1′ribose carbon in the common nucleosides.
Phosphorylated nucleosides are called nucleotides.
The most common bases in nucleotides are:
- The purines adenine and guanine;
- The pyrimidines cytosine, thymine, and uracil; and
- The pyridine nicotinamide.
The sugar component is either ribose or deoxyribose. (“Deoxy” simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.) Depending on their base sugar, nucleotides are therefore known as “deoxyribonucleotides” or “ribonucleotides.” The nucleic acid DNA is built of nucleotides with a deoxyribose sugar, whereas RNA contains nucleotides composed of ribose sugars.
See also
- Arabinose
- Lyxose
- Ribose
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.