Fossil fuel
Fossil fuels or mineral fuels are hydrocarbon fuels found within the top layer of the Earth’s crust. They range from highly volatile materials, such as methane, to liquid petroleum, to solids composed of almost pure carbon, such as anthracite coal.
The term "fossil fuels" is based on the widely accepted theory that they were formed from the fossilized remains of dead plants and animals,[1] during exposure to heat and pressure in the Earth's crust over hundreds of millions of years.[2] This process of formation is known as the biogenic theory. An opposing theory, called the abiogenic theory, maintains that the more volatile hydrocarbons, especially natural gas, were produced from nonliving materials.
Fossil fuels are of great importance because they can be burned (oxidized to carbon dioxide and water), producing significant amounts of energy. They are also the main source of raw materials for the petrochemical industry.
The Energy Information Administration estimated that in 2005, 86 percent of primary energy production in the world came from burning fossil fuels.[3] The remaining sources of energy were hydroelectricity at 6.3 percent, nuclear power at 6.0 percent, and other sources (geothermal, solar, wind, wood, and waste) at 0.9 percent.
Fossil fuels are considered non-renewable resources because they take millions of years to develop and reserves are being depleted much faster than new ones are being formed. Concerns about fossil fuel supplies have been among the reasons for regional and global tensions and conflicts. The production and excessive use of fossil fuels have also raised environmental concerns. For example, it has been estimated that the burning of fossil fuels produces around 6.3 billion metric tons (or 6.3 gigatons) of carbon dioxide per year, but natural processes can absorb only about half that amount.[4] It is argued that excessive production of carbon dioxide, a greenhouse gas, contributes to global warming. A global movement toward the generation of renewable energy is therefore under way to help meet increased energy needs.
Origins of fossil fuels
The origin of fossil fuels has been explained in different ways. Most petroleum geologists favor what is called the "biogenic theory," which holds that fossil fuels were formed from the remains of living organisms. (This view is the basis for calling the fuels, "fossil fuels.") An alternative theory, called the "abiogenic theory," holds that fossil fuels were formed from nonliving matter by mainly inorganic processes.
Biogenic theory
The biogenic hypothesis for the formation of petroleum was first proposed in 1757, by Russian scholar Mikhail Lomonosov. Since then, it has undergone several modifications.
According to the biogenic theory, petroleum was formed from the preserved remains of prehistoric zooplankton and algae that settled to the sea (or lake) bottom in large quantities under anoxic conditions. Over geological time, this organic matter, mixed with mud, was buried under heavy layers of sediment. The organic matter then underwent chemical changes—through the action of heat and pressure or the action of anaerobic bacteria—to form a waxy material called kerogen, which is found in various oil shales around the world.
As the source rock was buried deeper, overburden pressure raised temperatures into the oil window, between 60 and 120°C, in which the kerogen molecules were broken down into the straight-chain hydrocarbons that make up most of petroleum. Once crude oil formed, it became very fluid and migrated upward through the rock strata. This setting is called oil expulsion. Eventually it was either trapped in an oil reservoir or oil escaped to the surface and was biodegraded by soil bacteria.
Any oil buried deeper entered the gas window of 120°C to 220°C and was converted into natural gas by thermal cracking. Thus, below a certain depth, the theory predicts that no oil will be found, only unassociated gas. If it went even deeper, even natural gas would be destroyed by high temperatures.
By contrast, it is thought that coal was formed from the remains of terrestrial plants. In support of this view, many coal fields date to the carboniferous period.
Abiogenic theory
According to the theory of "abiogenic petroleum origin," natural petroleum was formed from deep carbon deposits, perhaps dating to the formation of the Earth. The ubiquity of hydrocarbons in the Solar System is taken as evidence that there may be a great deal more petroleum on Earth than commonly thought, and that petroleum may originate from carbon-bearing fluids that migrate upward from the mantle.
Various abiogenic hypotheses were first proposed in the nineteenth century, most notably by the Russian chemist Dmitri Mendeleev and the French chemist Marcellin Berthelot. Since then, these hypotheses have lost ground to the dominant view that petroleum is a fossil fuel. Abiogenic hypotheses saw a revival in the last half of the twentieth century by Russian and Ukrainian scientists, and more interest was generated in the West after the publication, in 1999, of The Deep Hot Biosphere by Thomas Gold. Gold's version of the hypothesis is based partly on the existence of a biosphere composed of thermophile bacteria in the Earth's crust, which may explain the existence of certain biomarkers in extracted petroleum.[5]
Although the abiogenic theory, according to Gold, is widely accepted in Russia, where it was intensively developed in the 1950s and 1960s, the vast majority of Western petroleum geologists consider the biogenic theory of petroleum formation scientifically proven.
Although evidence exists for the abiogenic creation of methane and hydrocarbon gases within the Earth,[6] it is argued that they are not produced in commercially significant quantities, and essentially all hydrocarbon gases that are extracted for use are thought to be biogenic in origin. Moreover, it is argued that there is no direct evidence to date of petroleum (liquid crude oil and long-chain hydrocarbon compounds) formed abiogenically within the crust, which is the essential prediction of the abiogenic petroleum theory.
The abiogenic origin of petroleum (liquid hydrocarbon oils) has recently been reviewed in detail by Glasby,[7] who raises a number of objections to the theory.
Uses
The use of coal as a fuel predates recorded history. Semisolid hydrocarbons from seeps were also burned in ancient times, but these materials were mostly used for waterproofing and embalming.[8] Commercial exploitation of petroleum, largely as a replacement for oils from animal sources (notably whale oil) for use in oil lamps began in the nineteenth century.[9] Natural gas, once flared-off as an unneeded byproduct of petroleum production, is now considered a very valuable resource.[10] Heavy crude oil, which is very much more viscous than conventional crude oil, and tar sands, where bitumen is found mixed with sand and clay, are becoming more important as sources of fossil fuel.[11] Oil shale and similar materials are sedimentary rocks containing kerogen, a complex mixture of high-molecular weight organic compounds which yields synthetic crude oil when heated (pyrolyzed), but they have not yet been exploited commercially.
Prior to the latter half of the eighteenth century, windmills or watermills provided the energy needed for industry, such as milling flour, sawing wood, or pumping water and burning wood or peat provided domestic heat. The wide scale use of fossil fuels, coal at first and petroleum later, to fire steam engines, enabled the Industrial Revolution. At the same time, gas lights using natural gas or coal gas were coming into wide use. The invention of the internal combustion engine and its use in automobiles and trucks greatly increased the demand for gasoline and diesel oil, both made from fossil fuels. Other forms of transportation, railways and aircraft, also required fossil fuels. The other major use for fossil fuels is in generating electricity.
Fossil fuels are also the main source of raw materials for the petrochemical industry.
Limits and alternatives
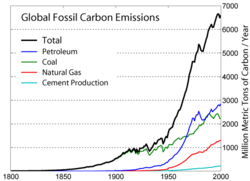
The principle of supply and demand suggests that as hydrocarbon supplies diminish, prices will rise. Therefore, higher prices will lead to increased alternative, renewable energy supplies, as previously uneconomic sources become sufficiently economical to exploit. Artificial gasolines and other renewable energy sources currently require more expensive production and processing technologies than conventional petroleum reserves, but may become economically viable in the near future.
Different alternative sources of energy include alcohols, hydrogen, nuclear, hydroelectric, solar, wind, and geothermal.
Levels and flows
Levels of primary energy sources are the reserves in the ground. Flows are production. The most important part of primary energy sources are the carbon based fossil energy sources. Oil, coal, and gas stood for 79.6 percent of primary energy production during 2002 (in million metric tons[12] of oil equivalent, MTOE) (34.9+23.5+21.2).
- Oil: 1,050,691 to 1,277,702 billion barrels (167 to 203 km³) 2003-2005
- Gas: 6,040,208 - 6,805,830 billion cubic feet (171,040 to 192,720 km³) 6,805.830*0.182= 1,239 BBOE 2003-2005
- Coal: 1,081,279 million short tons[15] (1,081,279*0.907186*4.879= 4,786 BBOE) (2004)
Flows (daily production) during 2002 (7.9 is a ratio to convert metric tons of oil equivalent to barrels of oil equivalent)
- Oil: (10,230*0.349)*7.9/365=77 MBD
- Gas: (10,230*0.212)*7.9/365=47 MBOED
- Coal: (10,230*0.235)*7.9/365=52 MBOED
Years of production left in the ground with the most optimistic reserve estimates (Oil & Gas Journal, World Oil)
- Oil: 1,277,702/77/365=45 years
- Gas: 1,239,000/47/365=72 years
- Coal: 4,786,000/52/365=252 years
Note that this calculation assumes that the product could be produced at a constant level for that number of years and that all of the reserves could be recovered. In reality, consumption of all three resources has been increasing. While this suggests that the resource will be used up more quickly, in reality, the production curve is much more akin to a bell curve. At some point in time, the production of each resource within an area, country, or globally will reach a maximum value, after which, the production will decline until it reaches a point where is no longer economically feasible or physically possible to produce.
The above discussion emphasizes worldwide energy balance. It is also valuable to understand the ratio of reserves to annual consumption (R/C) by region or country. For example, energy policy of the United Kingdom recognizes that Europe's R/C value is 3.0, very low by world standards, and exposes that region to energy vulnerability. Specific alternatives to fossil fuels are a subject of intense debate worldwide.
Environmental effects
In the United States, more than 90 percent of greenhouse gas emissions come from the combustion of fossil fuels.[16] Combustion of fossil fuels also produces other air pollutants, such as nitrogen oxides, sulphur dioxide, volatile organic compounds, and heavy metals.
According to Environment Canada:
The electricity sector is unique among industrial sectors in its very large contribution to emissions associated with nearly all air issues. Electricity generation produces a large share of Canadian nitrogen oxides and sulphur dioxide emissions, which contribute to smog and acid rain and the formation of fine particulate matter. It is the largest uncontrolled industrial source of mercury emissions in Canada. Fossil fuel-fired electric power plants also emit carbon dioxide, which may contribute to climate change. In addition, the sector has significant impacts on water and habitat and species. In particular, hydro dams and transmission lines have significant effects on water and biodiversity.[17]
Combustion of fossil fuels generates sulphuric, carbonic, and nitric acids, which fall to Earth as acid rain, impacting both natural areas and the built environment. Monuments and sculptures made from marble and limestone are particularly vulnerable, as the acids dissolve calcium carbonate.
Fossil fuels also contain radioactive materials, mainly uranium and thorium, that are released into the atmosphere. In 2000, about 12,000 metric tons of thorium and 5,000 metric tons of uranium were released worldwide from burning coal.[18] It is estimated that during 1982, U.S. coal burning released 155 times as much radioactivity into the atmosphere as the Three Mile Island incident.[19]
Burning coal also generates large amounts of bottom ash and fly ash. These materials are used in a wide variety of applications, utilizing, for example, about 40 percent of the U.S. production.[20]
Harvesting, processing, and distributing fossil fuels can also create environmental problems. Coal mining methods, particularly mountaintop removal and strip mining, have extremely negative environmental impacts, and offshore oil drilling poses a hazard to aquatic organisms. Oil refineries also have negative environmental impacts, including air and water pollution. Transportation of coal requires the use of diesel-powered locomotives, while crude oil is typically transported by tanker ships, each of which requires the combustion of additional fossil fuels.
Environmental regulation uses a variety of approaches to limit these emissions, such as command-and-control (which mandates the amount of pollution or the technology used), economic incentives, or voluntary programs.
An example of such regulation in the U.S. is the "EPA is implementing policies to reduce airborne mercury emissions. Under regulations issued in 2005, coal-fired power plants will need to reduce their emissions by 70 percent by 2018."[21]
In economic terms, pollution from fossil fuels is regarded as a negative externality. Taxation is considered one way to make societal costs explicit, in order to "internalize" the cost of pollution. This aims to make fossil fuels more expensive, thereby reducing their use and the amount of pollution associated with them, along with raising the funds necessary to counteract these factors. Although European nations impose some pollution taxes, they also give billions of subsidies to the fossil fuel industry, offsetting the taxes.
Many in America believe that a move away from an economy that is solely dependent on fossil fuels will allow a more even-handed approach to foreign policy. Former CIA Director James Woolsey recently outlined the national security arguments in favor of moving away from fossil fuels. Video of Woolsey speech
Fossil sources
Fossil sources can be used to produce fuels and plastics. Template:Secstub
Notes
- ↑ Irene Novaczek, Canada's Fossil Fuel Dependency. Retrieved December 5, 2007.
- ↑ EPA, Fossil fuel. Retrieved December 5, 2007.
- ↑ Energy Information Administration, International Energy Annual 2005. Retrieved December 5, 2007.
- ↑ Energy Information Administration, US Department of Energy on greenhouse gases. Retrieved December 5, 2007.
- ↑ Gold, Thomas. The Deep, Hot Biosphere (Copernicus Books, 1999). ISBN 0-387-98546-8
- ↑ Sherwood Lollar, et al., Abiogenic formation of alkanes in the Earth's crust as a minor source for global hydrocarbon reservoirs, Nature, 416(2002):522-524.
- ↑ G.P. Glasby, Abiogenic origin of hydrocarbons: An historical overview, Resource Geology 56: 83-96
- ↑ Zayn Bilkadi, Bells From the Sea : Ancient Oil Industries. Retrieved December 5, 2007.
- ↑ Max W. Ball, Douglas Ball, Daniel S. Turner, This Fascinating Oil Business (Indianapolis, IN: Bobbs-Merrill, 1965). ISBN 0-672-50829-X
- ↑ Rashad Kaldany, Global Gas Flaring Reduction: A Time for Action! Retrieved December 5, 2007.
- ↑ PR Log, Oil Sands Global Market Potential 2007. Retrieved December 5, 2007.
- ↑ A metric ton, also spelled "tonne," equals 1,000 kg.
- ↑ World Energy Reserves. Energy Information Administration. Retrieved July 10, 2008.
- ↑ International Data. Energy Information Administration. Retrieved July 10, 2008.
- ↑ A short ton equals 2,000 pounds (907.18474 kg).
- ↑ US EPA, Climate Change. Retrieved December 7, 2007.
- ↑ Environment Canada, Electricity Generation. Retrieved December 7, 2007.
- ↑ Alex Gabbard, Coal Combustion: Nuclear Resource or Danger. Retrieved December 7, 2007.
- ↑ Gordon J. Aubrecht, II, Nuclear proliferation through coal burning. Retrieved December 7, 2007.
- ↑ American Coal Ash Association, CCP Production and Use Survey. Retrieved December 7, 2007.
- ↑ Energystar, Frequently Asked Questions, Information on Proper Disposal of Compact Fluorescent Light Bulbs (CFLs). Retrieved December 7, 2007.
ReferencesISBN links support NWE through referral fees
- Jaccard, Mark. 2006. Sustainable Fossil Fuels: The Unusual Suspect in the Quest for Clean and Enduring Energy. New York: Cambridge University Press. ISBN 0521679796
- Gold, Thomas and Freeman Dyson. 1999. The Deep Hot Biosphere. New York: Copernicus. ISBN 0387985468
- Pfeiffer, Dale Allen. 2006. Eating Fossil Fuels: Oil, Food And the Coming Crisis in Agriculture. Gabriola Island, BC: New Society Publishers. ISBN 0865715653
External links
All links retrieved April 20, 2017.
- Williams, James L., A.F. Alhajji. The Coming Energy Crisis? WTRG Economics.
- Parfit, Michael. Powering the Future National Geographic.
- Gold, Thomas. The Origin of Methane (and Oil) in the Crust of the Earth. Internet Archives.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.

