Difference between revisions of "Alpha decay" - New World Encyclopedia
m (Alpha particle moved to Alpha decay: New title will be consistent with other articles named "Beta decay" and "Radioactive decay." Also, it combines text from Wiki articles "Alpha particle" and "Alpha decay.") |
Rosie Tanabe (talk | contribs) |
||
| (19 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{copyedited}} |
{{Nuclear physics}} | {{Nuclear physics}} | ||
| − | + | ||
| − | '''Alpha decay''' is a type of [[ | + | '''Alpha decay''' is a type of [[radioactive decay]] in which an [[atomic nucleus]] emits an '''[[alpha particle]].''' An alpha particle (or α particle, named after the first letter of the [[Greek alphabet]]) consists of two [[proton]]s and two [[neutron]]s bound together. It is identical to the nucleus of a [[helium]] atom and may therefore be written as He<sup>2+</sup> or <sup>4</sup><sub>2</sub>He. |
| − | For example: | + | |
| − | :<math> | + | Alpha decay is a form of [[nuclear fission]] in which the parent atom splits into two daughter products. When an atomic nucleus releases an alpha particle, the atom decays (is transformed) into another atom with a [[atomic weight|mass number]] that is lower by 4 and an [[atomic number]] that is lower by 2. For example, the alpha decay of radium atoms converts them to [[radon]] atoms, released as a gas. Also, most of the [[helium]] produced on [[Earth]] comes from the alpha decay of underground deposits of [[mineral]]s containing [[uranium]] or [[thorium]]. The helium is brought to the surface as a by-product of [[natural gas]] production. |
| + | |||
| + | Radioisotopes that emit alpha particles are used to provide safe power sources for certain types of [[generator]]s in [[space probe]]s and [[Artificial pacemaker|artificial heart pacemakers]]. The isotope [[americium#Isotopes|americium-241]] is an alpha-particle emitter and is used in some types of [[Smoke detector|smoke detector]]s. | ||
| + | {{toc}} | ||
| + | [[Image:Alpha Decay.svg|thumb|left|Alpha decay.]] | ||
| + | Alpha radiation that is external to the body is generally not harmful because the particles are absorbed by a few centimeters of [[air]] or by the thin layer of dead cells on the [[skin]]. However, if an alpha-radiating substance enters the body by ingestion, inhalation, or other means, some of the body's internal tissues receive a high dose of ionizing radiation, causing significant damage. | ||
| + | |||
| + | == Example of alpha decay == | ||
| + | |||
| + | A uranium-238 atom may decay to a thorium-234 atom, with the release of an alpha particle. This process may be written in either of two forms: | ||
| + | #<math> | ||
{}^2{}^{38}_{92}\hbox{U}\;\to\;{}^2{}^{34}_{90}\hbox{Th}\;+\;{}^4_2\hbox{He}^{2+}, | {}^2{}^{38}_{92}\hbox{U}\;\to\;{}^2{}^{34}_{90}\hbox{Th}\;+\;{}^4_2\hbox{He}^{2+}, | ||
</math> | </math> | ||
| − | + | #<math> | |
| − | |||
{}^{238}\hbox{U}\;\to\;^{234}\hbox{Th}\;+\;\alpha. | {}^{238}\hbox{U}\;\to\;^{234}\hbox{Th}\;+\;\alpha. | ||
</math> | </math> | ||
| − | |||
| − | + | The second form is preferred because the first form appears electrically unbalanced. Fundamentally, the recoiling thorium nucleus is quickly stripped of two electrons that may neutralize the alpha particle (helium cation). Alternatively, alpha particles may extract electrons from atoms in their immediate environment, ionizing those atoms. | |
| − | |||
| − | + | == Theoretical explanation == | |
| − | |||
| − | + | In the classical view, an alpha particle does not have enough energy to escape from the nucleus. (It is said to be trapped in a "potential well," or energy minimum.) By 1928, [[George Gamow]] solved the mystery of alpha decay by the theory known as "[[quantum tunneling]]." Applying the principles of [[quantum mechanics]], Gamow showed that an alpha particle has a tiny (but non-zero) probability of "tunneling" through the energy barrier and escaping from the nucleus. | |
| − | + | Unlike [[beta decay]], alpha decay is governed by the [[strong nuclear force]], which holds protons and neutrons together. Emission of an alpha particle sometimes leaves the atomic nucleus in an excited (higher energy) state. To remove the excess energy, the nucleus may emit a [[gamma ray]]. | |
| − | + | == Properties of alpha particles == | |
| − | + | Alpha particles are a highly [[ionizing radiation|ionizing]] form of [[particle radiation]], but they have low penetration. They are easily stopped by a sheet of paper. | |
| − | [[ | + | When an alpha particle is emitted, the [[atomic mass]] of an element goes down by roughly 4.0015 [[unified atomic mass unit|u]], due to the loss of 2 [[neutrons]] and 2 protons. The [[atomic number]] of the atom goes down by 2, as a result of the loss of 2 protons; the atom becomes a new element. An example of this is when radium becomes [[radon]] gas due to alpha decay. |
| − | + | [[Image:Alphaparticlemagnetic.svg|thumb|250px|An alpha particle is deflected by a magnetic field.]] | |
| + | [[Image:Alfa_beta_gamma_radiation.svg|thumb|250px|Alpha radiation consists of [[helium-4]] nuclei and is readily stopped by a sheet of paper. Beta radiation, consisting of [[electron]]s, is halted by an aluminum plate. Gamma radiation is eventually absorbed as it penetrates a dense material.]] | ||
| − | + | The alpha particle mass is 6.644656×10<sup>-27</sup> kg, which is equivalent to the energy of 3.72738 [[giga|G]][[electron volt|eV]]. The charge of an alpha particle is equal to +2e, where e is the magnitude of charge on an electron. | |
| − | + | The kinetic energy of alpha particles varies, with higher energy particles being emitted from larger nuclei. Most alpha particles have kinetic energies in the range of 3 to 7 MeV, which is a substantial amount of energy for a single particle. However, their high mass means alpha particles have a lower speed (with a typical kinetic energy of 5 MeV the speed is 15,000 km/s) than any other common type of radiation (such as [[Beta particle|β particles]], [[Gamma ray|γ rays]], or [[neutron radiation|neutrons]]). | |
| − | + | Alpha particles have a typical kinetic energy of 5 MeV (that is ≈0.13 percent of their total energy, i.e. 110 TJ/kg) and a speed of 15,000 km/s. This corresponds to a speed of around 0.05 [[Speed of light|c]], where c is the speed of light in a vacuum. Because of their relatively large mass, +2 charge, and relatively low velocity, they are very likely to interact with other atoms and lose their energy, so they are effectively absorbed within a few centimeters of air. | |
| − | + | Because of their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimeters in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 [[micrometer]]s, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body (most often because radioactive material has been inhaled or ingested), it is the most destructive form of [[ionizing radiation]]. It is the most strongly ionizing, and with large enough doses can cause any or all of the symptoms of [[radiation poisoning]]. It is estimated that [[chromosome]] damage from alpha particles is about 100 times greater than that caused by an equivalent amount of other radiation. The alpha emitter [[polonium-210]] is suspected of playing a role in [[lung cancer|lung]] and [[bladder cancer]] related to [[tobacco smoking]]. | |
| + | Because alpha particles occur naturally, but can have [[energy]] high enough to participate in a [[nuclear reaction]], study of them led to much early knowledge of [[nuclear physics]]. The physicist [[Ernest Rutherford]] famously used alpha particles to infer that [[J. J. Thomson]]'s [[Plum pudding model]] of the atom was fundamentally flawed. Rutherford's famous gold foil experiment was conducted by his students [[Hans Geiger]] and [[Ernest Marsden]]. A narrow beam of alpha particles was set up, passing through very thin (only a few hundred atoms thick) gold foil. The alpha particles were detected by a [[zinc sulfide]] screen, which emits a flash of light upon an alpha particle collision. Rutherford hypothesized that, assuming the "plum pudding" model of the atom was correct, the positively charged alpha particles would be only slightly deflected, if at all, by the dispersed positive charge predicted. It was found that some of the alpha particles were deflected at much larger angles than expected, with some even bouncing back. Although most of the alpha particles went straight through as expected, Rutherford commented that the few particles that were deflected was akin to shooting a fifteen inch shell at tissue paper only to have it bounce off, again assuming the "plum pudding" theory was correct. It was soon determined that the positive charge of the atom was concentrated in a small area in the center of the atom, hence making the positive charge dense enough to deflect any positively charged alpha particles that happened to come close to what was later termed the nucleus (it was not known at the time that alpha particles were themselves nuclei, nor was the existence of [[proton]]s or [[neutron]]s known). Rutherford's experiment subsequently led to the [[Niels Bohr|Bohr]] [[Bohr model|model]] and later the modern wave-mechanical model of the atom. | ||
| − | + | Rutherford's work also improved on previous measurements of the ratio of an alpha particle's mass to charge, allowing him to deduce that alpha particles were helium nuclei.<ref>Alexander Hellemans and Bryan H. Bunch, ''The Timetables of Science: A Chronology of the Most Important People and Events in the History of Science'' (New York: Simon and Schuster, 1988). ISBN 0671621300</ref> | |
| − | |||
| − | |||
| − | |||
| − | + | In computer technology in 1978, "[[soft error]]s" were traced to alpha particles in [[Intel]]'s DRAM ([[dynamic random access memory]]) chips. The discovery led to strict control of radioactive elements in the packaging of semiconductor materials, and the problem was largely considered "solved." | |
| − | + | ==Uses== | |
| − | + | The isotope [[americium#Isotopes|americium-241]] emits alpha particles, and this property is used in some types of [[Smoke detector|smoke detector]]s. The alpha particles [[Ionization|ionize]] molecules in the air within a narrow gap, producing a small [[Electric current|current]]. This current can be easily interrupted by smoke particles. | |
| − | + | Alpha decay can provide a safe power source for [[radioisotope thermoelectric generator]]s used for [[space probe]]s and [[Artificial pacemaker|artificial heart pacemakers]]. Alpha decay is much more easily shielded against than other forms of radioactive decay. [[Plutonium-238]], for example, requires only 2.5 [[Millimetre|mm]] of [[lead]] shielding to protect against unwanted radiation. | |
| − | + | ==Toxicity== | |
| − | |||
| − | |||
| − | + | Generally, external alpha radiation is not harmful because alpha particles are completely absorbed by a few centimeters of air. Even touching an alpha source is usually not harmful; the thin layer of dead cells on the [[skin]] will absorb them. However, if a substance radiating alpha particles is somehow introduced into an organism (such as by ingestion, inhalation, injection, or shrapnel penetration), some of the organism's tissue becomes exposed to a high [[Equivalent dose|dose]] of ionizing radiation. In such cases, the alpha radiation causes significant damage to the cells. | |
| − | + | Radon is a naturally occurring, radioactive gas found in soil, rock, and sometimes groundwater. When [[radon]] gas is inhaled, some of the radon particles stick to the inner lining of the lung. The particles that remain continue to decay over time, emitting alpha particles which may damage cells in the lung tissue.<ref>U.S. Environmental Protection Agency, [http://www.epa.gov/ttn/atw/hlthef/radionuc.html Radionuclides (including Radon, Radium and Uranium).] Retrieved October 23, 2007.</ref> | |
| − | + | As noted above, certain types of [[smoke detector]]s contain a small amount of the alpha emitter [[americium#Isotopes|americium-241]]. This isotope is extremely dangerous if inhaled or ingested, but the danger is minimal if the source is kept sealed. Many municipalities have established programs to collect and dispose of old smoke detectors, rather than let them go into the general waste stream. | |
| − | + | [[Marie Curie]]'s death from leukemia at age 66, was likely caused by prolonged exposure to high doses of ionizing radiation.<ref>Health Physics Society, [http://www.hps.org/publicinformation/ate/q535.html Did Marie Curie die of a radiation overexposure?] Retrieved October 23, 2007.</ref> Curie worked extensively with radium, which decays into radon, along with other radioactive materials that emit [[beta decay|beta]] and [[gamma ray|gamma]] rays. The 2006 assassination of Russian dissident [[Alexander Litvinenko]] is thought to have been caused by [[Radiation poisoning|poisoning]] with [[polonium-210]], an alpha emitter. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Notes == | == Notes == | ||
| Line 76: | Line 73: | ||
==References== | ==References== | ||
| − | * Krane, Kenneth S. | + | * Krane, Kenneth S. and David Halliday. 1988. ''Introductory Nuclear Physics.'' New York: Wiley. ISBN 047180553X |
| − | + | * Martin, Brian. 2006. ''Nuclear and Particle Physics: An Introduction''. Hoboken, NJ: Wiley. ISBN 0470025328 | |
| − | * Martin, Brian. 2006. ''Nuclear and Particle Physics: An Introduction''. Hoboken, NJ: Wiley. ISBN 0470025328 | + | * Poenaru, D. N. 1996. ''Nuclear Decay Modes.'' Philadelphia: Institute of Physics. ISBN 0750303387 |
| − | + | * Seiden, Abraham. 2004. ''Particle Physics: A Comprehensive Introduction''. San Francisco: Addison Wesley. ISBN 0805387366 | |
| − | * Poenaru, D. N. 1996. ''Nuclear Decay Modes.'' | + | * Tipler, Paul and Ralph Llewellyn. 2002. ''Modern Physics,'' 4th ed. New York: W.H. Freeman. ISBN 0-7167-4345-0 |
| − | + | * Turner, James E. 1995. ''Atoms, Radiation, and Radiation Protection,'' 2nd ed. New York: Wiley. ISBN 0471595810 | |
| − | * Seiden, Abraham. 2004. ''Particle Physics: A Comprehensive Introduction''. San Francisco | ||
| − | + | == External links == | |
| + | All links retrieved July 23, 2023. | ||
| − | * | + | * [http://education.jlab.org/glossary/alphadecay.html Alpha decay.] ''Jefferson Lab.'' |
{{Nuclear processes}} | {{Nuclear processes}} | ||
| Line 92: | Line 89: | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
[[Category:Physics]] | [[Category:Physics]] | ||
| − | |||
[[Category:Particle physics]] | [[Category:Particle physics]] | ||
{{credits|Alpha_particle|164335202|Alpha_decay|162770931}} | {{credits|Alpha_particle|164335202|Alpha_decay|162770931}} | ||
Latest revision as of 08:22, 23 July 2023
| Nuclear physics | ||||||||||||||
| ||||||||||||||
| Radioactive decay Nuclear fission Nuclear fusion
| ||||||||||||||
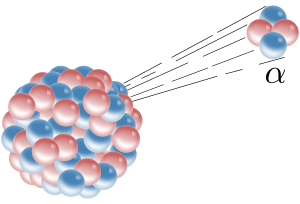
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle. An alpha particle (or α particle, named after the first letter of the Greek alphabet) consists of two protons and two neutrons bound together. It is identical to the nucleus of a helium atom and may therefore be written as He2+ or 42He.
Alpha decay is a form of nuclear fission in which the parent atom splits into two daughter products. When an atomic nucleus releases an alpha particle, the atom decays (is transformed) into another atom with a mass number that is lower by 4 and an atomic number that is lower by 2. For example, the alpha decay of radium atoms converts them to radon atoms, released as a gas. Also, most of the helium produced on Earth comes from the alpha decay of underground deposits of minerals containing uranium or thorium. The helium is brought to the surface as a by-product of natural gas production.
Radioisotopes that emit alpha particles are used to provide safe power sources for certain types of generators in space probes and artificial heart pacemakers. The isotope americium-241 is an alpha-particle emitter and is used in some types of smoke detectors.
Alpha radiation that is external to the body is generally not harmful because the particles are absorbed by a few centimeters of air or by the thin layer of dead cells on the skin. However, if an alpha-radiating substance enters the body by ingestion, inhalation, or other means, some of the body's internal tissues receive a high dose of ionizing radiation, causing significant damage.
Example of alpha decay
A uranium-238 atom may decay to a thorium-234 atom, with the release of an alpha particle. This process may be written in either of two forms:
The second form is preferred because the first form appears electrically unbalanced. Fundamentally, the recoiling thorium nucleus is quickly stripped of two electrons that may neutralize the alpha particle (helium cation). Alternatively, alpha particles may extract electrons from atoms in their immediate environment, ionizing those atoms.
Theoretical explanation
In the classical view, an alpha particle does not have enough energy to escape from the nucleus. (It is said to be trapped in a "potential well," or energy minimum.) By 1928, George Gamow solved the mystery of alpha decay by the theory known as "quantum tunneling." Applying the principles of quantum mechanics, Gamow showed that an alpha particle has a tiny (but non-zero) probability of "tunneling" through the energy barrier and escaping from the nucleus.
Unlike beta decay, alpha decay is governed by the strong nuclear force, which holds protons and neutrons together. Emission of an alpha particle sometimes leaves the atomic nucleus in an excited (higher energy) state. To remove the excess energy, the nucleus may emit a gamma ray.
Properties of alpha particles
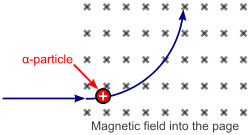
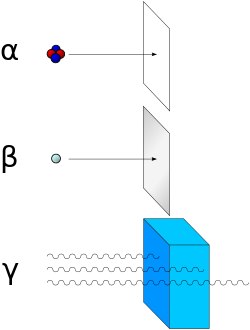
Alpha particles are a highly ionizing form of particle radiation, but they have low penetration. They are easily stopped by a sheet of paper.
When an alpha particle is emitted, the atomic mass of an element goes down by roughly 4.0015 u, due to the loss of 2 neutrons and 2 protons. The atomic number of the atom goes down by 2, as a result of the loss of 2 protons; the atom becomes a new element. An example of this is when radium becomes radon gas due to alpha decay.
The alpha particle mass is 6.644656×10-27 kg, which is equivalent to the energy of 3.72738 GeV. The charge of an alpha particle is equal to +2e, where e is the magnitude of charge on an electron.
The kinetic energy of alpha particles varies, with higher energy particles being emitted from larger nuclei. Most alpha particles have kinetic energies in the range of 3 to 7 MeV, which is a substantial amount of energy for a single particle. However, their high mass means alpha particles have a lower speed (with a typical kinetic energy of 5 MeV the speed is 15,000 km/s) than any other common type of radiation (such as β particles, γ rays, or neutrons).
Alpha particles have a typical kinetic energy of 5 MeV (that is ≈0.13 percent of their total energy, i.e. 110 TJ/kg) and a speed of 15,000 km/s. This corresponds to a speed of around 0.05 c, where c is the speed of light in a vacuum. Because of their relatively large mass, +2 charge, and relatively low velocity, they are very likely to interact with other atoms and lose their energy, so they are effectively absorbed within a few centimeters of air.
Because of their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimeters in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 micrometers, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body (most often because radioactive material has been inhaled or ingested), it is the most destructive form of ionizing radiation. It is the most strongly ionizing, and with large enough doses can cause any or all of the symptoms of radiation poisoning. It is estimated that chromosome damage from alpha particles is about 100 times greater than that caused by an equivalent amount of other radiation. The alpha emitter polonium-210 is suspected of playing a role in lung and bladder cancer related to tobacco smoking.
Because alpha particles occur naturally, but can have energy high enough to participate in a nuclear reaction, study of them led to much early knowledge of nuclear physics. The physicist Ernest Rutherford famously used alpha particles to infer that J. J. Thomson's Plum pudding model of the atom was fundamentally flawed. Rutherford's famous gold foil experiment was conducted by his students Hans Geiger and Ernest Marsden. A narrow beam of alpha particles was set up, passing through very thin (only a few hundred atoms thick) gold foil. The alpha particles were detected by a zinc sulfide screen, which emits a flash of light upon an alpha particle collision. Rutherford hypothesized that, assuming the "plum pudding" model of the atom was correct, the positively charged alpha particles would be only slightly deflected, if at all, by the dispersed positive charge predicted. It was found that some of the alpha particles were deflected at much larger angles than expected, with some even bouncing back. Although most of the alpha particles went straight through as expected, Rutherford commented that the few particles that were deflected was akin to shooting a fifteen inch shell at tissue paper only to have it bounce off, again assuming the "plum pudding" theory was correct. It was soon determined that the positive charge of the atom was concentrated in a small area in the center of the atom, hence making the positive charge dense enough to deflect any positively charged alpha particles that happened to come close to what was later termed the nucleus (it was not known at the time that alpha particles were themselves nuclei, nor was the existence of protons or neutrons known). Rutherford's experiment subsequently led to the Bohr model and later the modern wave-mechanical model of the atom.
Rutherford's work also improved on previous measurements of the ratio of an alpha particle's mass to charge, allowing him to deduce that alpha particles were helium nuclei.[1]
In computer technology in 1978, "soft errors" were traced to alpha particles in Intel's DRAM (dynamic random access memory) chips. The discovery led to strict control of radioactive elements in the packaging of semiconductor materials, and the problem was largely considered "solved."
Uses
The isotope americium-241 emits alpha particles, and this property is used in some types of smoke detectors. The alpha particles ionize molecules in the air within a narrow gap, producing a small current. This current can be easily interrupted by smoke particles.
Alpha decay can provide a safe power source for radioisotope thermoelectric generators used for space probes and artificial heart pacemakers. Alpha decay is much more easily shielded against than other forms of radioactive decay. Plutonium-238, for example, requires only 2.5 mm of lead shielding to protect against unwanted radiation.
Toxicity
Generally, external alpha radiation is not harmful because alpha particles are completely absorbed by a few centimeters of air. Even touching an alpha source is usually not harmful; the thin layer of dead cells on the skin will absorb them. However, if a substance radiating alpha particles is somehow introduced into an organism (such as by ingestion, inhalation, injection, or shrapnel penetration), some of the organism's tissue becomes exposed to a high dose of ionizing radiation. In such cases, the alpha radiation causes significant damage to the cells.
Radon is a naturally occurring, radioactive gas found in soil, rock, and sometimes groundwater. When radon gas is inhaled, some of the radon particles stick to the inner lining of the lung. The particles that remain continue to decay over time, emitting alpha particles which may damage cells in the lung tissue.[2]
As noted above, certain types of smoke detectors contain a small amount of the alpha emitter americium-241. This isotope is extremely dangerous if inhaled or ingested, but the danger is minimal if the source is kept sealed. Many municipalities have established programs to collect and dispose of old smoke detectors, rather than let them go into the general waste stream.
Marie Curie's death from leukemia at age 66, was likely caused by prolonged exposure to high doses of ionizing radiation.[3] Curie worked extensively with radium, which decays into radon, along with other radioactive materials that emit beta and gamma rays. The 2006 assassination of Russian dissident Alexander Litvinenko is thought to have been caused by poisoning with polonium-210, an alpha emitter.
Notes
- ↑ Alexander Hellemans and Bryan H. Bunch, The Timetables of Science: A Chronology of the Most Important People and Events in the History of Science (New York: Simon and Schuster, 1988). ISBN 0671621300
- ↑ U.S. Environmental Protection Agency, Radionuclides (including Radon, Radium and Uranium). Retrieved October 23, 2007.
- ↑ Health Physics Society, Did Marie Curie die of a radiation overexposure? Retrieved October 23, 2007.
ReferencesISBN links support NWE through referral fees
- Krane, Kenneth S. and David Halliday. 1988. Introductory Nuclear Physics. New York: Wiley. ISBN 047180553X
- Martin, Brian. 2006. Nuclear and Particle Physics: An Introduction. Hoboken, NJ: Wiley. ISBN 0470025328
- Poenaru, D. N. 1996. Nuclear Decay Modes. Philadelphia: Institute of Physics. ISBN 0750303387
- Seiden, Abraham. 2004. Particle Physics: A Comprehensive Introduction. San Francisco: Addison Wesley. ISBN 0805387366
- Tipler, Paul and Ralph Llewellyn. 2002. Modern Physics, 4th ed. New York: W.H. Freeman. ISBN 0-7167-4345-0
- Turner, James E. 1995. Atoms, Radiation, and Radiation Protection, 2nd ed. New York: Wiley. ISBN 0471595810
External links
All links retrieved July 23, 2023.
- Alpha decay. Jefferson Lab.
| |||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.