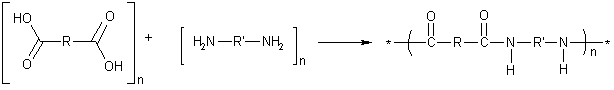Polymer
A polymer (from the Greek words polys, meaning "many," and meros, meaning "parts") is a chemical compound consisting of large molecules, each of which is a long chain made up of small structural units that are linked together by covalent chemical bonds. Each structural unit, called a monomer (Greek word monos means "alone" or "single"), is a small molecule of low-to-moderate molecular weight. Within a given polymer molecule, the monomers are usually identical or similar in structure. The chemical reaction by which monomers are linked together to form polymers is called polymerization.
Polymers form a large, diverse group of materials. Within each living organism, polymers (biopolymers) such as DNA, RNA, proteins, and polysaccharides perform specific functions that enable the organism to survive, grow, and reproduce. In addition, natural polymersâsuch as cotton, flax, jute, silk, and woolâhave long been used for the production of clothing, rope, carpeting, felt, insulation, and upholstery. More recently, scientists have discovered how to produce new polymers with a wide range of properties, at relatively low cost. Their work has given birth to a proliferation of plastics, artificial fibers, and synthetic rubber. Consequently, synthetic polymers are being used for numerous products in homes, schools, offices, factories, recreational facilities, and means of transportation and communication. Thus, artificial polymers have become an integral part of our modern technological society.
On the downside, most artificial polymers are not biodegradable, and factories and incineration furnaces often release chemical pollutants. To help solve these problems, recycling programs have been instituted in many countries, and manufacturing plants and incinerators are now fitted with pollutant traps. In addition, biodegradable polymers are being sought out.
General characteristics and classification
Most polymers are organicâthat is, their long chains have backbones of mostly carbon atoms. There are also some inorganic polymers, such as the silicones, which have a backbone of alternating silicon and oxygen atoms.
Polymer chains may or may not be cross-linked with one another. Thus the molecules of a polymer can have various topologies (shapes), such as linear (unbranched), branched, network (cross-linked 3-dimensional structure), comb, or star. The properties of a polymer depend on these shapes and on the structures of the monomers that make up the chains. For example, branched polymer chains cannot line up as close to one another as linear chains can. As a result, intermolecular bonds between branched chains are weaker, and such materials have lower densities, lower melting points, and lower tensile strength. Also, properties such as the solubility, flexibility, and strength of the polymer vary according to the types of monomers in the chains.
Polymers are typically classified as follows:
- Thermoplastics: A thermoplastic is a material that is deformable, melts to a liquid when heated, and freezes to a brittle, glassy state when cooled sufficiently. Most thermoplastics are polymers whose molecules have linear or branched structures. The molecules associate with one another through various interactions: weak van der Waals forces, as in the case of polyethylene and polypropylene; stronger dipole-dipole interactions; hydrogen bonding, as in the case of nylon; or the stacking of aromatic rings, as in the case of polystyrene.
- Thermosets (or thermosetting plastics): These are materials that are taken through a "curing" process with the addition of energy. The energy may be in the form of heat (generally above 200 °C), a chemical reaction, or irradiation. Thermoset materials are usually liquidy, powdery, or malleable prior to curing, and designed to be molded into their final form or used as adhesives. During the curing process, molecules of the starting material become cross-linked and take on a stronger form. Once cured, the thermoset cannot be remelted and remolded. Examples of thermosets are vulcanized rubber, Bakelite⢠(used in electrical insulators), melamine (used in worktop surfaces), and epoxy resin (used as an adhesive).
- Elastomers: The term elastomer is applied to an "elastic polymer"âthat is, a polymer that returns to its original shape when a load is removed. Elastomers are usually thermosets (that require curing), but some are thermoplastic. The long polymer chains become cross-linked during curing and account for the flexible nature of the material. The molecular form of elastomers has been likened to a "spaghetti and meatball" structure, where the meatballs signify cross-links between the flexible spaghetti strands (polymer chains). Most elastomers are rubbers, and the term elastomer is often used interchangeably with the term rubber. Examples of thermoplastic elastomers are HytrelÂŽ and SantopreneÂŽ.
- Coordination polymers: In a coordination polymer, many metal centers are interconnected through ligand bridges. Most of the common halides and oxides are coordination polymers. In a more conventional sense, the term coordination polymer is reserved for compounds where the metals are bridged by polyatomic ligands, such as cyanide and carboxylates. One of the most popular bridging ligands used in the synthesis of these polymers is a tricarboxylic acid called BTC (benzene-1,3,5-tricarboxylic acid). The polymers are metal salts of this acid. Another coordination polymer is Prussian Blue, which is based on Fe-CN-Fe linkages.
- Biopolymers (biological polymers): Biopolymers are a special class of polymers produced within living organisms. They include starch, proteins, peptides, DNA, and RNA. Their monomer units are sugars, amino acids (for proteins and peptides), and nucleotides (for DNA and RNA). Unlike synthetic (artificially produced) polymers, each biopolymer has a well-defined structure. Many biopolymers spontaneously fold into characteristic shapes that determine their biological functions.
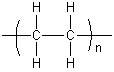
Synthetic polymers are often named after the monomer from which they are made. For example, polyethene (also called polyethylene) is the name given to the polymer formed when thousands of ethene (ethylene) molecules are bonded together. The polyethene molecules are straight or branched chains of repeating -CH2-CH2- units (with a -CH3 at each terminus). The polymerization reaction can be written as follows.
The product may also be written as:
By contrast, biopolymers have been named apart from their monomeric constitution. For instance, proteins are polymers of amino acids. Typically, each protein chain is made up of hundreds of amino acid monomers, and the sequence of these monomers determines its shape and biological function.
Whereas polyethylene forms spontaneously under the right conditions, the synthesis of biopolymers such as proteins and nucleic acids requires the help of specialized biological machinery, including enzymes that catalyze the reactions. Unlike synthetic polymers, these biopolymers (other than carbohydrates) have exact sequences and lengths. Since the 1950s, catalysts have also revolutionized the development of synthetic polymers. By allowing more careful control over polymerization reactions, polymers with new propertiesâsuch as the ability to emit colored lightâhave been manufactured.
Copolymerization
Copolymerization involves the linking together of two or more different monomers, producing chains with varied properties. For example, a protein can be called a copolymerâone in which different amino acid monomers are linked together. Depending on the sequence of amino acids, the protein chains have different shapes and functions.
When ethene is copolymerized with small amounts of 1-hexene (or 4-methyl-1-pentene), the product is called linear low-density polyethene (LLDPE). The C4 branches resulting from the hexene lower the density and prevent large crystalline regions from forming in the polymer, as they do in high-density polyethene (HDPE). This means that LLDPE can withstand strong tearing forces while maintaining flexibility.
The polymerization reaction may be carried out in a stepwise manner, to produce a structure with long sequences (or blocks) of one monomer alternating with long sequences of the other. The product is called a block copolymer.
In the case of some copolymers, called graft copolymers, entire chains of one kind (such as polystyrene) are made to grow out of the sides of chains of another kind (such as polybutadiene). The resultant product is less brittle and more impact-resistant. Thus, block and graft copolymers can combine the useful properties of both constituents and often behave as quasi-two-phase systems.
The formation of nylon is an example of step-growth polymerization, or condensation polymerization. The two types of monomers can have different R and R' groups, shown in the diagram below. The properties of the nylon can vary, depending on the R and R' groups in the monomers used.
The first commercially successful, completely synthetic polymer was nylon 6,6, with four carbon atoms in the R group (adipic acid) and six carbon atoms in the R' group (hexamethylene diamine). Each monomer actually contributes 6 carbon atoms (including the two carboxyl carbons of adipic acid)âhence the name nylon 6,6. In naming nylons, the number of carbons from the diamine is given first, and the number from the diacid, second. Kevlar is an aromatic nylon in which both R and R' are benzene rings.
Copolymers illustrate the point that the repeating unit in a polymerâsuch as a nylon, polyester, or polyurethaneâis often made up of two (or more) monomers.
Physical properties of polymers
Polymer chains have markedly unique physical properties, as follows.
- Molar mass distribution: During a polymerization reaction, polymer chains terminate after varying degrees of chain lengthening. The reaction produces an ensemble of differing chain lengths of differing molecular masses, with a (Gaussian) distribution around an average value. The molar mass distribution in a polymer describes this distribution of molecular masses for different chain lengths. Biopolymers, however, have well-defined structures, and they therefore do not have a molar mass distribution.
- Degree of polymerization: This is the number of monomer units in an average polymer chain, at time t in a polymerization reaction. For most industrial purposes, synthetic polymer chains need to have thousands or tens of thousands of monomer units.
- Crystallinity, and thermal phase transitions:
- (a) Melting point (Tm): Thermoplastic (non-cross-linked) polymers have a melting temperature above which their crystalline structure entirely disappears.
- (b) Glass transition temperature (Tg): The glass transition temperature of a material is the temperature below which its molecules have little relative mobility. This temperature is usually applicable to glasses and plastics that have wholly or partially amorphous phases. Thermoplastic (non-cross-linked) polymers have a Tg value below which they become rigid and brittle, and can crack and shatter under stress. (The Tg value is lower than Tm.) Above Tg, the polymer becomes rubbery and capable of deformation without fracture. This is one of the properties that make many plastics useful. Such behavior, however, is not exhibited by cross-linked thermosetting plasticsâonce cured, they are set for life, never deforming or melting when heated.
- Stereoregularity (or tacticity): This property describes the arrangement of functional groups on the backbone of carbon chains.
Chemical properties of polymers
The attractive forces between polymer chains play a large part in determining a polymer's properties. Given that polymer chains are so long, these interchain forces are amplified far beyond the attractions between conventional molecules. Also, longer chains are more amorphous (randomly oriented). Polymers can be visualized as tangled spaghetti chainsâthe more tangled the chains, the more difficult it is to pull any one strand out. These stronger forces typically result in high tensile strength and melting points.
The intermolecular forces in polymers are determined by dipoles in the monomer units. For example, polymers containing amide groups can form hydrogen bonds between adjacent chains. The somewhat positively charged hydrogen atoms in the N-H groups of one chain are strongly attracted to the somewhat negatively charged oxygen atoms in the C=O groups on another. Such strong hydrogen bonds are responsible for the high tensile strength and melting point of Kevlar.
In the case of polyesters, there is dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in C-H groups. Dipole bonding is not as strong as hydrogen bonding, so the polyester's melting point and strength are lower than Kevlar's, but polyesters have greater flexibility.
If one considers polyethene, the monomer units (ethene) have no permanent dipole. Attractive forces between polyethene chains arise from weak van der Waals forces. Molecules can be thought of as being surrounded by a cloud of negative electrons. As two polymer chains approach, their electron clouds repel one another. This has the effect of lowering the electron density on one side of a polymer chain, creating a slight positive charge on this side. This charge is enough to attract the second polymer chain. Van der Waals forces are quite weak, however, so polyethene melts at low temperatures.
Applications
Applications of synthetic polymers
- Acrylonitrile butadiene styrene (ABS): This is a common thermoplastic, appropriate for making light but rigid products such as automotive body parts, protective head gear, golf club heads, and LEGOÂŽ toys.
- Polyacrylates (acrylic): Noted for their transparency and resistance to breakage, polyacrylates may be used as substitutes for window glass. A familiar product in this group is PlexiglasÂŽ.
- Cellulose acetate: It is used as a film base in photography, as a component in some adhesives, and as a synthetic fiber. The fiber form is used for dresses, draperies, upholstery, diapers, cigarette filters and other filters, and fiber-tip pens.
- Ionomers: These are useful for golf ball covers, semipermeable membranes, dental cements, and fuel cells.
- Liquid crystal polymers: Uses for this group of polymers include electrical and electronic applications, automotive parts, and engineering parts.
- Polyamides, such as nylon and KevlarÂŽ: Nylon fibers are used in clothing, parachutes, ropes, carpets, guitar and racket strings, and fishing nets. KevlarÂŽ is used in applications ranging from bicycles to bulletproof jackets.
- Polyesters, such as polyethylene terephthalate (PET) and polycarbonates: Polyester fibers are used to make fabrics for personal clothing, bed sheets, bedspreads, curtains, and so forth. In addition, polyesters are used to make bottles, films, liquid crystal displays, holograms, filters, and electrical insulation. Thermosetting polyester resins are commonly used as casting materials, fiberglass laminating resins, and nonmetallic auto-body fillers. Polyesters are also widely used as a finish on high-quality wooden products like guitars, pianos, and vehicle or yacht interiors.
- Polytetrafluoroethylene (TeflonÂŽ): Among its many uses, it is suitable as an insulator in cables and connector assemblies and as a material for printed circuit boards (at microwave frequencies), bearings, bushings, and gears.
- Polyethylene (polyethene, PE): The polyethylenes are a widely used group of materials and are classified according to their molecular weight, density, and branching. For instance, ultra high molecular weight PE (UHMWPE) is used for can- and bottle-handling machine parts, moving parts on weaving machines, bearings, gears, artificial joints, and the newer bulletproof vests. High-density PE (HDPE) is used in making milk jugs, detergent bottles, margarine tubs, and garbage containers. Low-density PE (LDPE) is used for film wrap and plastic bags, as well as for some rigid containers.
- Melamine resin: Combined with formaldehyde, it produces a thermoset plastic that is used to make decorative wall panels, laminates, kitchen utensils, and plates. It is the main constituent of FormicaÂŽ and ArboriteÂŽ.
- Epoxy resin: It is used for many applications, including coatings, adhesives, and composite materials, such as those using carbon fiber and fiberglass reinforcements.
- Polybutadiene (BR): This synthetic rubber has a high resistance to wear and is used mainly for the manufacture of tires.
- Polychloroprene (Neoprene): This synthetic rubber has many applications, such as for wetsuits, electrical insulation, car fan belts, gaskets, hoses, corrosion-resistant coatings, and as padding in metal cases.
Applications of biopolymers
- Cotton: This soft fiber, which grows around the seeds of the cotton plant (Gossypium species), consists of nearly pure cellulose. It is most often spun into thread and used to make a soft, breathable textile, the most widely used natural fiber in clothing today.
- Flax: Flax fibers have been used for the production of linen for 5,000 years. The best grades are used for fabrics such as damasks, lace, and sheeting. Coarser grades are used for manufacturing twine and rope. Flax fiber is also a raw material for the high-quality paper used for banknotes.
- Hemp: Hemp fibers, obtained from the Cannabis species of plants, are used to make cordage and clothing.
- Jute: Jute fibers, composed of plant cellulose and lignin, are used to make coarse fabrics (called burlap or hessian cloth) and sacks (called gunny bags).
- Kenaf: Kenaf fibers, made by the kenaf plant (Hibiscus cannabinus), are used for the manufacture of rope, twine, coarse cloth, and paper.
- Silk: This protein fiber, obtained from the cocoons of silkworm larvae, is woven into textiles.
- Wool: This protein fiber, derived mainly from the fur of sheep and goats, is used for making clothing, carpeting, felt, insulation, and upholstery. It is also used to absorb odors and noise in heavy machinery and stereo speakers.
- Zein: This protein, found in maize, is used in the manufacture of textile fibers, biodegradable plastics, printing inks, and adhesives. It is also used as a coating for candy, nuts, fruit, and encapsulated foods and drugs.
Natural functions of biopolymers
- Proteins. There are different types of proteins that are involved in numerous functions in each living cell. Examples include:
- Catalysis of biochemical reactions, carried out by numerous enzymes
- Transport and storage of small molecules and ions
- Immune defense, such as by forming antibodies
- Sending and receiving signals, such as by receptors on cell surfaces
- Structural support, such as components of the skin, hair, and bone.
- Coordinated motion, such as the components of muscles and molecular motors.
- Control of cell growth, such as by factors that control the synthesis of messenger RNA and proteins.
- RNA (ribonucleic acid). There are different types of RNA that carry out different functions. Examples include:
- messenger RNA (mRNA): Various mRNAs get their information from DNA and serve as templates for the synthesis of proteins.
- transfer RNA (tRNA): Specific tRNA molecules carry specific amino acids and transfer them to growing protein chains.
- ribosomal RNA (rRNA): rRNA molecules are part of cellular structures called ribosomes, which function as "workbenches" on which proteins are synthesized.
- ribozymes: These are RNA molecules that can function as enzymes, that is, they can catalyze chemical reactions.
- small interfering RNA (siRNA): Among their various functions, siRNAs are involved in pathways by which they interfere with the expression of specific genes.
- DNA (deoxyribonucleic acid). A constituent of the chromosomes (and organelles such as mitochondria and chloroplasts) of living cells, DNA serves as an "informational" molecule and genetic material that is inherited. Its known functions include:
- Carrier of information for RNA structures.
- Carrier of information for protein structures.
- Replication, so that it can be passed down from one generation to the next.
- Polysaccharides. These large, polymeric carbohydrates occur in different types and serve various functions. Examples are as follows.
- Cellulose: It is a common material that provides structure for plant cell walls.
- Starch: It is a combination of two polysaccharides (amylose and amylopectin) and is made by plants to store excess glucose.
- Glycogen ("animal starch"): This polysaccharide is the main storage form of glucose in animal and human cells.
Examples of thermoplastics
- Acrylonitrile butadiene styrene (ABS)
- Celluloid
- Cellulose acetate
- Ethylene vinyl acetate (EVA)
- Ethylene vinyl alcohol (EVAL)
- Fluoroplastics (including polytetrafluoroethylene (PTFE), or TeflonÂŽ)
- Ionomers
- Kydexâ˘, an acrylic/PVC alloy
- Liquid crystal polymer (LCP)
- Polyacetal (POM or Acetal)
- Polyacrylates (Acrylic or Acrylates)
- Polyacrylonitrile (PAN or Acrylonitrile)
- Polyamide (PA) (including nylon and KevlarÂŽ)
- Polyamide-imide (PAI)
- Polyaryletherketone (PAEK or Ketone)
- Polybutadiene (PBD)
- Polybutylene (PB)
- Polycyclohexylene dimethylene terephthalate (PCT)
- Polyhydroxyalkanoates (PHAs)
- Polyketone (PK)
- Polyester (including polycarbonate (PC), polyethylene terephthalate (PET), polybutylene terephthalate (PBT), polylactic acid (PLA))
- Polyethylene (PE)
- Polyetheretherketone (PEEK)
- Polyetherimide (PEI)
- Polyethersulfone (PES)- see Polysulfone
- Polyethylenechlorinates (PEC)
- Polyimide (PI)
- Polymethylpentene (PMP)
- Polyphenylene oxide (PPO)
- Polyphenylene sulfide (PPS)
- Polyphthalamide (PPA)
- Polypropylene (PP)
- Polystyrene (PS)
- Polysulfone (PSU)
- Polyvinyl chloride (PVC)
- Spectralon
Examples of thermosets
- Vulcanized rubber
- Bakelite⢠(a phenol formaldehyde resin, used in electrical insulators and plastic wear)
- Duroplast
- Urea-formaldehyde foam (used in plywood, particleboard, and medium-density fibreboard)
- Melamine resin (used on worktop surfaces)
- Polyester resin (used in glass-reinforced plastics/fiberglass)
- Epoxy resin (used as an adhesive and in fibre-reinforced plastics such as glass-reinforced plastic and graphite-reinforced plastic)
Examples of elastomers
Unsaturated rubbers that can be cured by sulfur vulcanization
- Natural rubber (NR)
- Polyisoprene (IR)
- Butyl rubber (copolymer of isobutylene and isoprene, IIR)
- Halogenated butyl rubbers: chloro butyl rubber (CIIR), bromo butyl rubber (BIIR)
- Polybutadiene (BR)
- Styrene-butadiene rubber (SBR, copolymer of polystyrene and polybutadiene)
- Nitrile rubber (NBR, copolymer of polybutadiene and acrylonitrile), also called buna N rubbers
- Hydrated nitrile rubbers (HNBR): TherbanÂŽ and ZetpolÂŽ
- Chloroprene rubber (CR): polychloroprene, Neoprene, Baypren
Saturated rubbers that cannot be cured by sulfur vulcanization
- Ethylene propylene rubber (EPM, a copolymer of polyethylene and polypropylene)
- Ethylene propylene diene rubber (EPDM, a combination of polyethylene, polypropylene, and a diene)
- Epichlorohydrin rubber (ECO)
- Polyacrylic rubber (ACM, ABR)
- Silicone rubber (SI, Q, VMQ)
- Fluorosilicone rubber (FVMQ)
- Fluoroelastomers (FKM, FPM): VitonÂŽ, TecnoflonÂŽ, FluorelÂŽ, Dai-ElÂŽ
- Perfluoroelastomers (FFKM)
- Tetrafluoro ethylene/propylene rubbers (FEPM)
- Chlorosulfonated polyethylene (CSM): HypalonÂŽ
- Ethylene-vinyl acetate (EVA)
Other types of elastomers
- Thermoplastic Elastomers (TPE): HytrelÂŽ, SantopreneÂŽ
- Polyurethane rubber
- Resilin, Elastin
- Polysulfide rubber
See also
ReferencesISBN links support NWE through referral fees
- Budinski, Kenneth G., and Michael K. Budinski. Engineering Materials: Properties and Selection. Prentice Hall College Div, 2001. ISBN 0130305332
- Lodge, Timothy P. Polymer Chemistry. CRC Press, 1920. ISBN 978-1466581647
- Young,Robert J., and Peter A. Lovell. Introduction to Polymers. CRC Press, 2011. ISBN 978-0849339295
External links
All links retrieved November 24, 2022.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Polymer history
- Thermoplastic history
- Thermosetting_plastic history
- Elastomer history
- Coordination_polymers history
- Biopolymer history
- Glass_transition_temperature history
- Polyester history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.