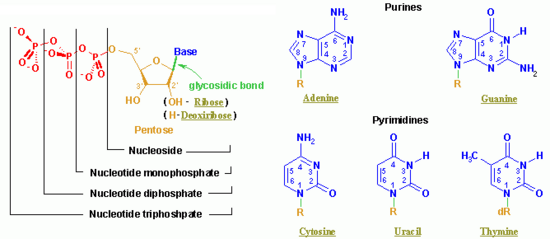
Nucleotide
A nucleotide is a chemical compound with three components: a nitrogen-containing base, a pentose (five-carbon) sugar (relatively simple carbohydrates), and one or more phosphate groups. Although best known as the structural units of the nucleic acids DNA and RNA, which store and transfer genetic information in organisms, nucleotides participate in nearly all biochemical processes.
The ubiquitous presence of nucleotides from viruses and bacteria to humans reflects a common base and unity among all living organisms despite the remarkable diversity of life.
Nucleotides play a variety of key roles in cellular metabolism:
- ATP, an adenine nucleotide, is a universal energy currency in the cells of biological systems.
- Adenine nucleotides are components of three major coenzymes, NAD+, FAD, and CoA, organic molecules that assist in various biochemical reactions by serving as carriers.
- Nucleotides also function as regulators of metabolism. Cyclic AMP is a ubiquitous mediator of the action of many hormones that regulate the breakdown or synthesis of biomolecules in a particular tissue or organ. Covalent modifications introduced by ATP alter the activities of many enzymes.
Chemical structure and nomenclature
The nitrogen-containing base of a nucleotide (also called the nucleobase) is typically a derivative of either purine or pyrimidine, which are heterocyclic compounds (organic compounds that contain a ring structure that has, in addition to carbon, such atoms as sulfur, oxygen, or nitrogen). The most common bases in nucleotides are:
- The purines adenine and guanine;
- The pyrimidines cytosine, thymine, and uracil; and
- The pyridine nicotinamide.
The sugar component is either deoxyribose or ribose. (âDeoxyâ simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.) Depending on their base sugar, nucleotides are therefore known as âdeoxyribonucleotidesâ or âribonucleotides.â The nucleic acid DNA (which stands for deoxyribonucleic acid) is built of nucleotides with a deoxyribose sugar, whereas RNA (or ribonucleic acid) contains nucleotides composed of ribose sugars.
Nucleotide names are abbreviated into standard three- or four-letter codes that indicate their structural components:
- The first letter is lower case and indicates whether the nucleotide in question is a deoxyribonucleotide (denoted by a "d") or a ribonucleotide (no letter).
- The second letter indicates the nucleoside corresponding to the base. Nucleosides resemble the structure of nucleotides (i.e., they contain a base bonded to a sugar) but lack the phosphate group. A nucleotide can thus also be defined as the phosphate ester of a nucleoside. (In chemistry, esters are organic compounds in which an organic group replaces a hydrogen atom or multiple hydrogens in an oxygen acid.) The abbreviations are as follows:
- G: Guanine
- A: Adenine
- T: Thymine
- C: Cytosine
- U: Uracil (which is not present in DNA, but takes the place of thymine in RNA)
- The third and fourth letters indicate the length of the attached phosphate chain (Mono-, Di-, Tri-) and the presence of a phosphate (P).
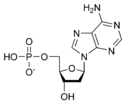
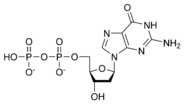
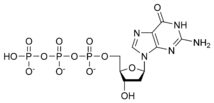
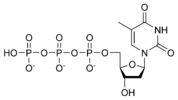
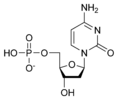
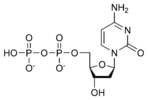
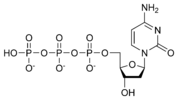
Thus, for example, deoxy-adenosine-triphosphate (pictured at right), one of the activated precursors in the synthesis of DNA, is abbreviated as dATP.
Nucleotides are the components of DNA and RNA
The nucleic acids DNA and RNA are polymers of nucleotide units; that is, they contain a large number of repeating nucleotide units connected by covalent chemical bonds. RNA molecules, for example, can contain as few as 75 nucleotides to more than five thousand nucleotides.
DNA consists of two helical deoxyribonucleotide chains coiled around a common axis. The chains run in opposite directions, and are held together by hydrogen bonds between pairs of bases from each chain. Adenine is always paired with thymine, and guanine with cytosine (i.e., a purine pairs with a pyrimidine).
Because pairing causes the nucleotide bases to face inward toward the helical axis, the sugar and phosphate groups of the nucleotides run along the outside; the two chains they form are sometimes called the backbones of the helix. In fact, it is chemical bonds between the phosphates and the sugars that link one nucleotide to the next in the DNA strand. Thus, the sugar-phosphate backbones play a primarily structural role.
In contrast, the nucleobases (which are the variable part of the nucleotide) carry genetic information. Within a gene, the sequence of nucleotides along a DNA strand defines a messenger RNA sequence, which in turn defines a protein. The relationship between the nucleotide sequence and the amino-acid sequence of the protein is determined by simple cellular rules of translation, known collectively as the genetic code. The genetic code is the relation between the sequence of bases in DNA (or its RNA transcript) and the sequence of amino acids in proteins. Amino acids are coded by groups of three bases (called codons) starting from a fixed point (e.g. ACT, CAG, TTT). These codons can then be translated with messenger RNA and then transfer RNA from the chemical language of nucleic acids to that of amino acids, with each codon corresponding to a particular amino acid.
There are two major differences between the nucleotide components of RNA and DNA: (1) the sugar units in RNA nucleotides are riboses rather than deoxyriboses and (2) one of the four major bases in RNA is uracil (U) instead of thymine (T).
Nucleotides function in cell metabolism
ATP is the universal energy currency of the cell
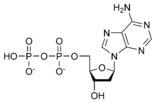
While ATP (adenosine triphosphate) is one of four nucleotides required for the synthesis of ribonucleic acids, it is primarily known in biochemistry for its role in metabolism as the "molecular currency" of intracellular energy transfer. As the name suggests, the structure of this nucleotide consists of a purine base (adenine), a ribose sugar, and three phosphate groups.
ATP is an energy-rich molecule because it contains two phosphohydride bonds between its three phosphate groups. A large amount of energy is released when the hydrolysis of these high energy phosphate-phosphate bonds is carried out. This energy can be used to power reactions such as the active transport of molecules across cell membranes, the synthesis of macromolecules (e.g., proteins) from simple components, and the mechanical work of muscle contractions.
The hydrolysis of ATP yields free inorganic Pi and adenosine diphosphate (ADP), which can be broken down further to another Pi and adenosine monophosphate (AMP). ATP can also be broken down to AMP directly, with the formation of PPi. ATP is in turn formed from ADP and Pi when fuel molecules are oxidized in chemotrophs or when light is trapped by phototrophs.
At any given moment, the total quantity of ATP in the human body is about 0.1 mole. The energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled two to three thousand times during a single day. ATP cannot be stored, hence its consumption must closely follow its synthesis.
Other nucleotide triphosphates with high-energy phosphate bonds may also power some biosynthetic reactions: namely, guanosine triphosphate (GTP), uradine triphosphate (UTP), and cytidine triphosphate (CTP).
Several nucleotides function as coenzymes
Coenzymes are non-protein, organic molecules that assist enzymes in catalyzing specific reactions. While some coenzymes undergo chemical changes during the course of a reaction (e.g., being reduced or oxidized), they must be returned to their original state once the reaction has been completed. A recurring set of nucleotides facilitates metabolic reactions; it includes:
- NAD+ (nicotinamide adenine dinucleotide), an important coenzyme found in cells. NADH is the reduced form of NAD+. The reducing potential (i.e., the ability to donate electrons) stored in NADH can be converted to ATP through the electron transport chain or used for anabolic metabolism.
The other major electron carrier in the oxidation of fuel molecules is FAD (flavin adenine dinucleotide).
- NADP (nicotinamide adenine dinucleotide phosphate), which is formed from NAD+ with the addition of a phosphate. NADP is used in anabolic reactions, such as fatty acid and nucleic acid synthesis, which require NADPH as a reducing agent. In chloroplasts, NADP is an oxidizing agent important in the preliminary reactions of photosynthesis. The NADPH produced by photosynthesis is then used as reducing power for the biosynthetic reactions in the Calvin cycle of photosynthesis.
- CoA (coenzyme A), notable for its role in the synthesis and oxidization of fatty acids and the oxidation of pyruvate in the citric acid cycle. Its main function is to carry acyl groups (such as the acetyl group) or thioesters. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA (where "A" stands for acetylation). Acetyl CoA has a high acetyl group-transfer potential, meaning that it carries an activated acetyl group, which it can deliver for degradation and energy generation or for biosynthesis.
Nucleotides also play roles in regulation and signaling
A common strategy of regulation involves the covalent attachment of phosphate groups to enzymes involved in metabolic reactions, which alters their catalytic activity. ATP donates one of its phosphate groups in these reactions, which are catalyzed by enzymes called protein kinases. This process, called phosphorylation, occurs within the cell, where ATP is abundant. It is a form of reversible covalent modification; phosphoryl groups may be removed by hydrolysis.
Cyclic adenosine monophosphate (cAMP or cyclic AMP), a molecule derived from ATP, transfers the effects of hormones like glucagon and adrenaline, which are first messengers that relay signals from one cell to another, to the intracellular environment. These hormones cannot get through the cell membrane, so cAMP serves as a second messenger, communicating their message within the cell. The regulatory effects of cAMP are achieved in eukaryotic cells by activating a specific protein kinase called PKA (protein kinase A). Cyclic AMP binds to specific locations on the two regulatory units of this enzyme, thus activating the catalytic units and enabling them to phosphorylate substrate proteins. cAMP controls many biological processes, including the decomposition of glycogen into glucose (glycogenolysis).
Examples of chemical structures
Nucleotides
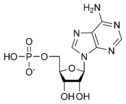 Adenosine monophosphate AMP |
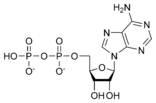 Adenosine diphosphate ADP |
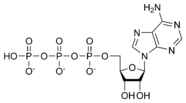 Adenosine triphosphate ATP |
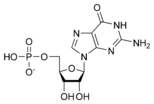 Guanosine monophosphate GMP |
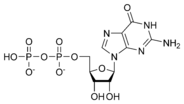 Guanosine diphosphate GDP |
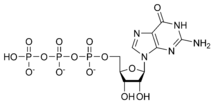 Guanosine triphosphate GTP |
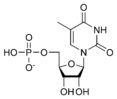 Thymidine monophosphate TMP |
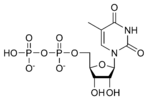 Thymidine diphosphate TDP |
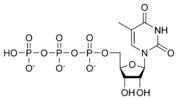 Thymidine triphosphate TTP |
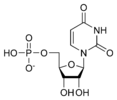 Uridine monophosphate UMP |
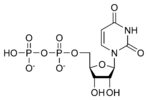 Uridine diphosphate UDP |
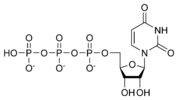 Uridine triphosphate UTP |
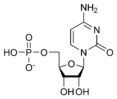 Cytidine monophosphate CMP |
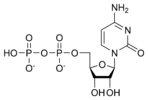 Cytidine diphosphate CDP |
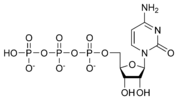 Cytidine triphosphate CTP |
Deoxynucleotides
Origin of nucleotides
One explanation for the near ubiquity of nucleotides in the chemical processes of life is the RNA world hypothesis, which posits that RNA evolved before DNA and proteins from free-floating nucleotides in the early "primordial soup." The hypothesis was aided in the 1980s by the discovery that certain RNA molecules (called ribozymes) may function as enzymes, whereas previously only proteins were believed to have catalytic ability. This discovery provided an explanation for how early RNA molecules might have first catalyzed their own replication and developed a range of enzymatic activities. Next, RNA molecules might have begun to catalyze the synthesis of proteins from amino acid molecules. Proteins are more versatile than nucleotides, as they can be built from 20 amino acids with unique side chains versus the four bases of nucleotides. Next, DNA might have been formed by reverse transcription of RNA, with DNA eventually replacing RNA as the storage form of genetic material because of the greater stability and dependability of its double helical structure. There are remaining difficulties with the RNA world hypothesis; however, the multifunctional nature of nucleotides does suggest the interconnectedness of life and its common origins.
ReferencesISBN links support NWE through referral fees
- Lindahl, T. 1993. âInstability and decay of the primary structure of DNA.â Nature 362 (6422): 709-715.
- Pääbo, S. 1993. âAncient DNA.â Scientific American 269 (5): 60-66.
- Stryer, L. 1995. Biochemistry, 4th edition. New York: W. H. Freeman.
- Watson, J. D., and F. H. C. Crick. 1953. âA structure for deoxyribose nucleic acidâ (PDF). Nature 171: 737-738.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Nucleotide history
- DNAÂ history
- Adenosine_triphosphate history
- Nicotinamide_adenine_dinucleotide history
- Coenzyme_AÂ history
- Cyclic_adenosine_monophosphate history
- RNA_world_hypothesis history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.