Polyester
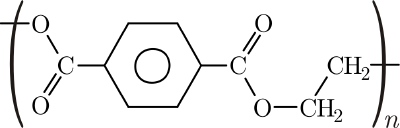
Polyester is the name for a class of polymers that contain the ester functional group in their main chain. Although some types of polyesters can be found in nature, such as the cutin of plant cuticles, the term polyester is usually applied to the artificially synthesized materials. Among the synthetic types of polyester, one of the most important is known as polyethylene terephthalate (PET); another is polycarbonate.
PET fibers are the most widely used manufactured fibers in the United States. Fabrics made from PET fibers are used for apparel and home furnishings. In addition, polyesters are used to make bottles, films, liquid crystal displays, filters, and electrical insulation. Some polyesters are thermosetting resins that are widely used in the bodies of automobiles and yachts.
Properties and uses
Thermoplastic polyesters, such as PET, may be heated and processed into different forms, including fibers, sheets, and three-dimensional shapes. Although combustible at high temperatures, polyester tends to shrink away from flames and often self-extinguishes. Woven PET fabrics are used for bed sheets, bedspreads, curtains, and draperies. Polyester fiberfill is also used to stuff pillows, comforters, and cushion padding.
Polyester fabrics sometimes have a "less natural" feel when compared to similarly woven fabrics made from natural fibers, such as cotton. However, polyester fabrics may exhibit other advantages over natural fabrics, particularly improved wrinkle resistance. For this reason, polyester fibers are often spun together with natural fibers, like cotton, to produce cloth with blended properties.
Polyesters are also used to make bottles, films, tarpaulin, liquid crystal displays, holograms, filters, dielectric film for capacitors, film insulation for wire, and insulating tapes.
Liquid crystalline polyesters are among the first such polymers to be used industrially. In general, they have extremely good mechanical properties and are very resistant to heat. They can therefore be used for seals in jet engines.
Thermosetting polyester resins are generally copolymers of unsaturated polyesters with styrene.[1] The unsaturation in the polyester is generally obtained by the use of maleic acid or fumaric acid, each of which has a carbon-carbon double bond in its molecular structure. Another important family is the group of vinyl esters. Here the unsaturation is found in the alcohol part of the polyester. The double bond of the unsaturated polyester reacts with styrene resulting in a 3-D crosslinked structure, the thermoset material. Unsaturated polyesters are commonly used as casting materials, fiberglass laminating resins, and non-metallic auto-body fillers. Fiberglass reinforced unsaturated polyesters find wide application in the bodies of yachts and automobiles.
Polyester is also widely used as a finish on high-quality wooden products like guitars, pianos and vehicle/yacht interiors.[2] The thixotropic properties of the sprayable form of polyester make it ideal for use on open-grain timbers, as it can quickly fill the grain and has a high build film thickness per coat. The cured polyester can then be sanded and polished to a high-gloss, durable finish.
Synthesis
Synthesis of polyesters is generally achieved by what are called polycondensation reactions.[3] Some examples of these reactions are given below.
Azeotrope esterification
In this classical method, an alcohol and a carboxylic acid react to form a carboxylic ester, with the release of water molecules. For example, to synthesize PET, ethylene glycol (an alcohol with two OH groups) and terephthalic acid (an acid with two carboxyl groups) may be used as the starting materials.
To assemble the polymer, the water formed by the reaction must be continually removed, by a process known as azeotrope distillation.
Alcoholic transesterification
In transesterification, the alkoxy group of an ester compound is exchanged for another alcohol. For example, PET can be synthesized by reacting ethylene glycol with dimethyl terephthalate (the dimethyl ester of terephthalic acid).
Shown below is the reaction between an ester-terminated oligomer (with the alkoxy group -OCH3) and an alcohol-terminated oligomer (with the OH group), producing a larger oligomer and methanol (CH3OH).
O \\ C - OCH3 + OH[Oligomer2] / [Oligomer1] |
O \\ C - O[Oligomer2] + CH3OH / [Oligomer1] | |
| (ester-terminated oligomer + alcohol-terminated oligomer) | (larger oligomer + methanol) |
Acylation (HCl method)
This method involves the use of the acid chloride (R-COCl) form of the acid. Thus the polycondensation proceeds with emission of hydrochloric acid (HCl) instead of water. This method can be carried out in solution or as an enamel.
Recycling PET bottles
Although all thermoplastics are technically recyclable, PET bottle recycling is more practical than many other plastic applications. The primary reason is that plastic carbonated soft drink bottles and water bottles are almost exclusively PET, which makes them more easily identifiable in a recycle stream. PET has a resin identification code of one. Like many other plastics, PET is also an excellent candidate for thermal recycling (incineration), as it is composed of carbon, hydrogen and oxygen with only trace amounts of catalyst elements (no sulfur), and it has the energy content of soft coal.
See also
Notes
- ↑ In organic chemistry, an "unsaturated" molecule is one that contains double or triple covalent bonds between carbon atoms.
- ↑ Burns Guitars, Rolls Royce, and Sunseeker are examples of companies that use polyester on their products.
- ↑ In a "condensation reaction," two molecules (or functional groups) combine to form a single molecule, accompanied by the loss of a small molecule, such as water.
ReferencesISBN links support NWE through referral fees
- Albertsson, Ann-Christine. Degradable Aliphatic Polyesters. Advances in Polymer Science, 157. Berlin: Springer, 2002. ISBN 3540422498
- Goodman, Isaac, and Brian Parkyn. Polyesters. London: Published for the Plastics Institute by Iliffe Books, 1965. OCLC 2033281
- Kadolph, Sara J. Textiles. Upper Saddle River, NJ: Pearson Prentice Hall, 2007. ISBN 9780131187696
- Scheirs, John, and Timothy E. Long (eds.). Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters. Wiley Series in Polymer Science. Hoboken, NJ: John Wiley & Sons, 2003. ISBN 0471498564
| |||||||||
| ||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.