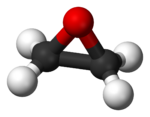
Ethylene oxide
- "Oxirane" redirects here.For oxiranes as a class of molecules, see Epoxide.
| Ethylene oxide | |
|---|---|
|
|
| IUPAC name | epoxyethane |
| Other names | ethylene oxide, dimethylene oxide, oxirane, oxacyclopropane |
| Identifiers | |
| Abbreviations | EO |
| CAS number | [] |
| PubChem | |
| EINECS number | |
| KEGG | |
| MeSH | |
| ChEBI | |
| RTECS number | KX2450000 |
| SMILES | C1CO1 |
| InChI | InChI=1/C2H4O/c1-2-3-1/h1-2H2 |
| Properties | |
| Molecular formula | C2H4O |
| Molar mass | 44.05 g mol−1 |
| Appearance | colorless gas |
| Density | 0.882 g/mL, 7.360 lbs/gallon |
| Melting point |
−111.3 °C |
| Boiling point |
10.7 °C |
| Solubility in water | miscible |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−52.6 kJ mol−1 |
| Standard molar entropy S |
243 J mol−1 K−1 |
| Hazards | |
| NFPA 704 |
|
| Flash point | −20 °C |
| Explosive limits | 3 to 100% |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
The chemical compound ethylene oxide (formula C2H4O) is an important industrial chemical used as an intermediate in the production of ethylene glycol and other chemicals, and as a sterilant for foods and medical supplies. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor. It is the simplest example of an epoxide.
The IUPAC name for ethylene oxide is epoxyethane. Other names for it include oxirane and dimethylene oxide.
History
Ethylene oxide was first prepared in 1859 by the French chemist Charles-Adolphe Wurtz.[1] He obtained it by treating 2-chloroethanol with a base. It achieved industrial importance during World War I as a precursor to both the coolant ethylene glycol and the chemical weapon mustard gas.
In 1931, Theodore Lefort, another French chemist, discovered a means to prepare ethylene oxide directly from ethylene and oxygen, using silver as a catalyst. Since 1940, this method has been the predominant means of production of ethylene oxide industrially.[2]
Production
For the industrial production of ethylene oxide, ethylene (H2C=CH2) is reacted with oxygen (O2) at 200–300 °C, in the presence of a silver catalyst (containing large silver nanoparticles) supported on alumina. Typically, chemical modifiers such as chlorine are also included. Pressures used for the reaction are in the neighborhood of 1-2 MPa. The chemical equation for this reaction is:
- H2C=CH2 + ½ O2 → C2H4O
The typical yield for this reaction under industrial conditions is 70-80 percent.
The above reaction takes place through an intermediate (oxametallacycle), leading to two possible reaction pathways, as follows.
- Ethylene oxide formation:
- H2C=CH2 + O → C2H4O
- Acetaldehyde formation:
- H2C=CH2 + O → CH3CHO
The latter pathway is the first step in complete combustion, which produces carbon dioxide and water:
- CH3CHO + 5/2 O2 → 2CO2 + 2H2O
In the laboratory, ethylene oxide can be conveniently produced by the action of an alkali hydroxide (OH−) on ethylene chlorohydrin (CH2OH−CH2Cl), as follows.[3]
- CH2OH−CH2Cl + OH− → C2H4O + Cl− + H2O
Note that ethylene chlorohydrin can be readily prepared by the action of hypochlorous acid (HOCl) on ethylene.
Reactions
Most reactions are ring openings by nucleophiles.
In industry, epoxyethane is reacted with water in the presence of a sulfuric acid catalyst. A tenfold molar excess of water is used to obtain ethylene glycol:
- C2H4O + H2O → HOCH2CH2OH
Despite the large excess of water, various types of polyethylene glycol (PEG) or polyethylene oxide (PEO) are still formed as secondary products. The degree of polymerization increases as a smaller proportion of water is used:
- n(CH2CH2O) + H2O → HO(CH2CH2O)nH
For example, under the right conditions it can give diethylene glycol (HOCH2CH2OCH2CH2OH), triethylene glycol, etc.
Similarly, reaction with ammonia can yield ethanolamine, diethanolamine, or triethanolamine.
Ethylene oxide is also important in the manufacture of surfactants and other detergents, in a process called ethoxylation.
One class of ethylene oxide derivatives that has attracted much scientific attention is the crown ethers, which are cyclic oligomers of ethylene oxide. These compounds have the ability to make ionic compounds such as salts soluble in nonpolar solvents which they otherwise could not dissolve in. However, the high cost of these compounds has largely confined their use to the laboratory rather than industrial practice.
Uses
Ethylene oxide gas kills bacteria (and their endospores), mold, and fungi, and can therefore be used to sterilize substances that would be damaged by sterilizing techniques such as pasteurization that rely on heat. Ethylene oxide sterilization for the preservation of spices was patented in 1938 by the American chemist Lloyd Hall, and it is still used in that role. Additionally, ethylene oxide is widely used to sterilize medical supplies such as bandages, sutures, and surgical implements. The overwhelming majority of medical items are sterilized with ethylene oxide. Preferred methods have been the traditional chamber sterilization method, where a chamber is flooded with a mix of ethylene oxide and other gases which are later aerated, and the more recent gas diffusion method developed in 1967 which relies on a bag that wraps the elements to be sterilized and acts a a mini-chamber in order to minimize gas consumption and make the process economically feasible for small loads. Other names for this alternative method for small loads are: Anprolene method, bag sterilization method or micro-dose sterilization method.
Most ethylene oxide, however, is used as an intermediate in the production of other chemicals. The major use of ethylene oxide is in the production of ethylene glycol. The primary end use for ethylene glycol is in the production of polyester polymers. Ethylene glycol is more commonly known for its use as an automotive coolant and antifreeze.
Because of its high flammability and wide explosive concentration range in air, ethylene oxide is sometimes used as the fuel component of a fuel-air explosive.
Health effects
Ethylene oxide is toxic by inhalation. Symptoms of overexposure include headache and dizziness, progressing with increasing exposure to convulsions, seizure and coma. It is also an irritant to skin and the respiratory tract, and inhaling the vapors may cause the lungs to fill with fluid several hours after exposure.[4]
Ethylene oxide is usually stored as a pressurized or refrigerated liquid. At room temperature and pressure, it rapidly evaporates, potentially causing frostbite in cases of skin exposure.
Laboratory animals exposed to ethylene oxide for their entire lives have had a higher incidence of liver cancer. However, studies on human beings who have worked with ethylene oxide for extended periods and may have experienced low doses during that time have found no increase in cancer risk. Chronic ethylene oxide exposure may increase the risk of cataracts in humans.
In animals, ethylene-oxide can cause numerous reproductive effects, including mutations and a higher rate of miscarriages. Its reproductive effects on humans have not been well studied, but it is considered probable that ethylene oxide exposure has similar effects on human reproduction.
Ethylene oxide is classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC).[5]
See also
- Acetaldehyde
- Epoxy
- Ethenol
Notes
- ↑ Wurtz, A. (1859). . Compt. rend. 48: 101-104.
- ↑ P. P. McClellan (1950). Manufacture and Uses of Ethylene Oxide and Ethylene Glycol. Ind. Eng. Chem. 42: 2402 - 2407.
- ↑ Streitwieser, Andrew, and Clayton H. Heathcock. 1976. Introduction to Organic Chemistry. New York: Macmillan. ISBN 0024180106.
- ↑ http://www.anpro.com/support/MSDS.pdf
- ↑ IARC Vol 60
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
- Streitwieser, Andrew, and Clayton H. Heathcock. 1976. Introduction to Organic Chemistry. New York: Macmillan. ISBN 0024180106.
External links
- National Pollutant Inventory - Ethylene oxide fact sheet
- Ethylene Oxide User's Guide
- Ethylene Oxide MSDS (Material Safety Data Sheet).
- National Institute for Occupational Safety and Health - Ethylene Oxide Topic Page
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
