Difference between revisions of "Earth's atmosphere" - New World Encyclopedia
m |
(imported latest version of article from Wikipedia) |
||
| Line 1: | Line 1: | ||
[[image:Edge_of_Space.png|thumb|right|175px|Layers of Atmosphere (NOAA)]] | [[image:Edge_of_Space.png|thumb|right|175px|Layers of Atmosphere (NOAA)]] | ||
| + | {{redirect|Air}} | ||
| + | '''Earth's atmosphere''' is a layer of gases surrounding the planet [[Earth]] and retained by the Earth's [[gravity]]. It contains roughly 78% [[nitrogen]] and 21% [[oxygen]], with trace amounts of [[#Composition|other gases]]. This mixture of gases is commonly known as '''air'''. The atmosphere protects [[life]] on [[Earth]] by absorbing [[ultraviolet]] [[solar radiation]] and reducing [[temperature]] extremes between [[day]] and [[night]]. | ||
| − | + | The atmosphere has no abrupt cut-off. It slowly becomes thinner and fades away into [[space]]. There is no definite boundary between the atmosphere and [[outer space]]. Three-quarters of the atmosphere's mass is within 11 km of the [[planet|planetary]] surface. In the [[United States]], persons who travel above an [[altitude]] of 50.0 miles (80.5 km) are designated as [[Astronaut|astronauts]]. An altitude of 120 km (75 mi or 400,000 ft) marks the boundary where atmospheric effects become noticeable during re-entry. The [[Karman line]], at 100 km (62 mi), is also frequently used as the boundary between atmosphere and [[space]]. | |
| − | |||
| − | The atmosphere has no abrupt cut-off. It slowly becomes thinner and fades away into space. There is no definite boundary between the atmosphere and [[outer space]]. Three-quarters of the atmosphere's mass is within 11 km of the [[planet|planetary]] surface. In the United States, persons who travel above an altitude of 50.0 miles (80.5 km) are designated as [[Astronaut|astronauts]]. An altitude of 120 km (75 mi or 400,000 ft) marks the boundary where atmospheric effects become noticeable during re-entry. The [[Karman line]], at 100 km (62 mi), is also frequently used as the boundary between atmosphere and space. | ||
==Temperature and the atmospheric layers== | ==Temperature and the atmospheric layers== | ||
The [[temperature]] of the Earth's atmosphere varies with [[altitude]]; the [[mathematical relationship]] between temperature and altitude varies between the different atmospheric layers: | The [[temperature]] of the Earth's atmosphere varies with [[altitude]]; the [[mathematical relationship]] between temperature and altitude varies between the different atmospheric layers: | ||
| − | * [[troposphere]]: From the Greek word | + | * [[troposphere]]: From the Greek word "tropos" meaning to turn or mix. The troposphere is the lowest layer of the atmosphere starting at the surface going up to between 7 km at the poles and 17 km at the equator with some variation due to weather factors. The troposphere has a great deal of vertical mixing due to solar heating at the surface. This heating warms air masses, which then rise to release [[latent heat]] as sensible heat that further buoys the air mass. This process continues until all water vapor is removed. In the troposphere, on average, temperature decreases with height due to [[Joule-Thomson effect|expansive cooling]]. |
| − | * [[stratosphere]]: from that | + | * [[stratosphere]]: from that 7–17 km range to about 50 km, temperature increasing with height. |
* [[mesosphere]]: from about 50 km to the range of 80 km to 85 km, temperature decreasing with height. | * [[mesosphere]]: from about 50 km to the range of 80 km to 85 km, temperature decreasing with height. | ||
| − | * [[thermosphere]]: from | + | * [[thermosphere]]: from 80–85 km to 640+ km, temperature increasing with height. |
The boundaries between these regions are named the [[tropopause]], [[stratopause]], and [[mesopause]]. | The boundaries between these regions are named the [[tropopause]], [[stratopause]], and [[mesopause]]. | ||
| − | The average temperature of the atmosphere at the surface of earth is 14 | + | The average temperature of the atmosphere at the surface of earth is 14 °C. |
===Various atmospheric regions=== | ===Various atmospheric regions=== | ||
Atmospheric regions are also named in other ways: | Atmospheric regions are also named in other ways: | ||
| − | * [[ionosphere]] | + | * [[ionosphere]] – the region containing [[ion]]s: approximately the mesosphere and thermosphere up to 550 km. |
| − | * [[exosphere]] | + | * [[exosphere]] – above the ionosphere, where the atmosphere thins out into [[outer space|space]]. This is the last major atmosphere. |
| − | * [[magnetosphere]] | + | * [[magnetosphere]] – the region where the [[Earth's magnetic field]] interacts with the [[solar wind]] from the [[Sun]]. It extends for tens of thousands of kilometers, with a long tail away from the Sun. |
| − | * [[ozone layer]] | + | * [[ozone layer]] – or ozonosphere, approximately 10 - 50 km, where [[stratosphere|stratospheric]] [[ozone]] is found. Note that even within this region, ozone is a minor constituent by volume. |
| − | * upper atmosphere | + | * upper atmosphere – the region of the atmosphere above the [[mesopause]]. |
| − | * [[Van Allen radiation belt]]s | + | * [[Van Allen radiation belt]]s – regions where [[Solar wind|particles]] from the Sun become concentrated. |
==Pressure== | ==Pressure== | ||
| + | :''Barometric Formula: (used for airplane flight) [[barometric formula]]'' | ||
:''Main article: [[Atmospheric pressure]]'' | :''Main article: [[Atmospheric pressure]]'' | ||
| − | Atmospheric pressure is a direct result of the weight of the air. This means that air pressure varies with location and time because the amount (and weight) of air above the earth varies with location and time. Atmospheric pressure drops by ~50% at an altitude of about 5 km (equivalently, about 50% of the total atmospheric mass is within the lowest 5 km). The average atmospheric pressure, at [[sea level]], is about 101.3 [[kilopascal]]s (about 14.7 pounds per square inch). | + | :''Nasa mathematical model: [[NRLMSISE-00]]'' |
| + | Atmospheric pressure is a direct result of the weight of the air. This means that air pressure varies with location and time, because the amount (and weight) of air above the earth varies with location and time. Atmospheric pressure drops by ~50% at an altitude of about 5 km (equivalently, about 50% of the total atmospheric mass is within the lowest 5 km). The average atmospheric pressure, at [[sea level]], is about 101.3 [[kilopascal]]s (about 14.7 pounds per square inch). | ||
| + | |||
| + | ==Thickness of the atmosphere== | ||
| + | Although the atmosphere exists at heights of 1000 km and more, it is so thin as to be considered nonexistant. | ||
| + | *57.8% of the atmosphere is below the summit of [[Mount Everest]]. | ||
| + | *72% of the atmosphere is below the common cruising altitude of commercial airliners (about 10000 m or 32800 ft). | ||
| + | *99.99999% of the atmosphere is below the highest [[North American X-15|X-15]] plane flight on [[August 22]], [[1963]], which reached an altitude of 354,300 ft or 108 km. | ||
| + | Therefore, most of the atmosphere (99.9999%) is below 100 km, although in the rarified region above this there are [[Aurora (astronomy)|aurora]]s and other atmospheric effects. | ||
==Composition== | ==Composition== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Minor components of air not listed above include: [[nitrous oxide]] | + | [[Image:Atmosphere_gas_proportions.gif|thumb|right|250px|Composition of Earth's atmosphere. The lower pie represents the least common gases that compose 0.038% of the atmosphere. Values normalized for illustration.]] |
| + | {| style="width:300px" | ||
| + | |+'''Composition of<br>dry atmosphere, by volume''' | ||
| + | |colspan=2 style="font-size: 85%" |''[[Parts per million|ppmv]]: parts per million by volume'' | ||
| + | |- | ||
| + | !align = "left" | '''Gas''' | ||
| + | !style="width:130px" align = "left" |'''Volume''' | ||
| + | |- | ||
| + | |[[Nitrogen]] (N<sub>2</sub>) ||780,840 ppmv (78.084%) | ||
| + | |- | ||
| + | |[[Oxygen]] (O<sub>2</sub>) ||209,460 ppmv (20.946%) | ||
| + | |- | ||
| + | |[[Argon]] (Ar) ||9,340 ppmv (0.9340%) | ||
| + | |- | ||
| + | |[[Carbon dioxide]] (CO<sub>2</sub>) ||350 ppmv | ||
| + | |- | ||
| + | |[[Neon]] (Ne) ||18.18 ppmv | ||
| + | |- | ||
| + | |[[Helium]] (He) ||5.24 ppmv | ||
| + | |- | ||
| + | |[[Methane]] (CH<sub>4</sub>) ||1.745 ppmv | ||
| + | |- | ||
| + | |[[Krypton]] (Kr) ||1.14 ppmv | ||
| + | |- | ||
| + | |[[Hydrogen]] (H<sub>2</sub>) ||0.55 ppmv | ||
| + | |- | ||
| + | |colspan=2 |'''Not included in above dry atmosphere:''' | ||
| + | |- | ||
| + | |[[Water vapor]] (highly variable) ||typically 1% | ||
| + | |} | ||
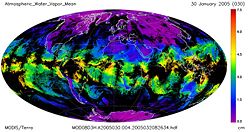
| + | [[Image:Atmospheric_Water_Vapor_Mean.2005.030.jpg|thumb|250px|right|Mean Atmospheric Water Vapor.]] | ||
| + | <font size=1>''Source for figures above: [http://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html NASA]. Carbon dioxide and methane updated (to 1998) by [[IPCC]] TAR table 6.1 [http://www.grida.no/climate/ipcc_tar/wg1/221.htm]. The NASA total was 17 ppmv over 100%, and CO<sub>2</sub> was increased here by 15 ppmv. To normalize, N<sub>2</sub> should be reduced by about 25 ppmv and O<sub>2</sub> by about 7 ppmv.''</font> | ||
| + | |||
| + | '''Minor components of air not listed above include:''' | ||
| + | {| style="width:300px" | ||
| + | !align = "left" | '''Gas''' | ||
| + | !style="width:300px" align = "left" |'''Volume''' | ||
| + | |- | ||
| + | |[[nitrous oxide]] ||0.5 ppmv | ||
| + | |- | ||
| + | |[[xenon]] ||0.09 ppmv | ||
| + | |- | ||
| + | |[[ozone]] ||0.0 to 0.07 ppmv | ||
| + | |- | ||
| + | |[[nitrogen dioxide]] ||0.02 ppmv | ||
| + | |- | ||
| + | |[[iodine]] ||0.01 ppmv | ||
| + | |- | ||
| + | |[[carbon monoxide]] ||trace | ||
| + | |- | ||
| + | |[[ammonia]] ||trace | ||
| + | |} | ||
| − | The mean molecular mass of air is 28.97 g/mol. | + | *The mean molecular mass of air is 28.97 g/mol. |
=== Heterosphere === | === Heterosphere === | ||
| − | Below an altitude of about 100 km, the Earth's atmosphere has a more-or-less uniform composition (apart from water vapor) as described above. However, above about 100 km, the Earth's atmosphere begins to have a composition which varies with altitude. This is essentially because, in the absence of mixing, the density of a gas falls off exponentially with increasing altitude, but at a rate which depends on the [[molecular mass]]. Thus higher mass constituents, such as oxygen and nitrogen, fall off more quickly than lighter constituents such as [[helium]], molecular [[hydrogen]], and atomic hydrogen. Thus there is a layer, called the '''heterosphere''', in which the earth's atmosphere has varying composition. As the altitude increases, the atmosphere is dominated successively by helium, molecular hydrogen, and atomic hydrogen. The precise altitude of the heterosphere and the layers it contains varies significantly with temperature.[http://www.oma.be/BIRA-IASB/Public/Research/Thermo/Thermotxt.en.html] | + | Below the [[turbopause]] at an altitude of about 100 km, the Earth's atmosphere has a more-or-less uniform composition (apart from water vapor) as described above; this constitutes the '''homosphere'''.[http://amsglossary.allenpress.com/glossary/search?id=homosphere1] However, above about 100 km, the Earth's atmosphere begins to have a composition which varies with altitude. This is essentially because, in the absence of mixing, the density of a gas falls off exponentially with increasing altitude, but at a rate which depends on the [[molecular mass]]. Thus higher mass constituents, such as oxygen and nitrogen, fall off more quickly than lighter constituents such as [[helium]], molecular [[hydrogen]], and atomic hydrogen. Thus there is a layer, called the '''heterosphere''', in which the earth's atmosphere has varying composition. As the altitude increases, the atmosphere is dominated successively by helium, molecular hydrogen, and atomic hydrogen. The precise altitude of the heterosphere and the layers it contains varies significantly with temperature.[http://web.archive.org/web/20041030214502/http://www.oma.be/BIRA-IASB/Public/Research/Thermo/Thermotxt.en.html] |
==Density and mass== | ==Density and mass== | ||
| − | The density of air at sea level is about 1.2 kg/m<sup>3</sup>. Natural variations of the [[barometric pressure]] occur at any one altitude as a consequence of [[weather]]. This variation is relatively small for inhabited altitudes but much more pronounced in the outer atmosphere and space due to variable solar radiation | + | :''Main article: [[Density of air]]'' |
| + | The density of air at sea level is about 1.2 kg/m<sup>3</sup>. Natural variations of the [[barometric pressure]] occur at any one altitude as a consequence of [[weather]]. This variation is relatively small for inhabited altitudes but much more pronounced in the outer atmosphere and space due to variable solar radiation. | ||
| + | |||
| + | |||
| + | [[Image:Atmosphere model.png|350px|thumb|left|Temperature and pressure against altitude from the [[NRLMSISE-00]] [[standard atmosphere]] model]] | ||
The atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the [[barometric formula]]. More sophisticated models are used by meteorologists and space agencies to predict weather and orbital decay of satellites. | The atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the [[barometric formula]]. More sophisticated models are used by meteorologists and space agencies to predict weather and orbital decay of satellites. | ||
| − | The | + | The average mass of the atmosphere is about 5,000 trillion metric tons. According to the National Center for Atmospheric Research, "The total mean mass of the atmosphere is 5.1480 x 10<sup>18</sup> kg with an annual range due to water vapor of 1.2 or 1.5 x 10<sup>15</sup> kg depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27 x 10<sup>16</sup> kg and the dry air mass as 5.1352 ±0.0003 x 10<sup>18</sup> kg." |
| − | |||
| − | The | ||
| − | + | The above composition percentages are done by volume. Assuming that the gases act like ideal gases, we can add the percentages p multiplied by their molar masses m, to get a total t = sum (p·m). Any element's percent by mass is then p·m/t. When we do this to the above percentages, we get that, by mass, the composition of the atmosphere is 75.523% nitrogen, 23.133% oxygen, 1.288% argon, 0.053% carbon dioxide, 0.001267% neon, 0.00029% methane, 0.00033% krypton, 0.000724% helium, and 0.0000038 % hydrogen. | |
== The evolution of the Earth's atmosphere == | == The evolution of the Earth's atmosphere == | ||
| + | {{seealso|History of Earth}} | ||
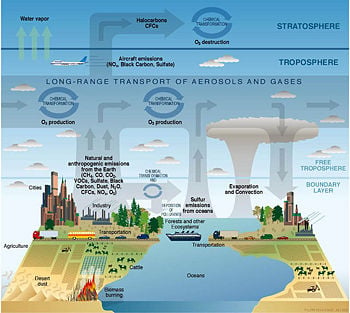
[[Image:Atmosphere composition diagram.jpg|thumb|right|350px|Diagram of chemical and transport processes related to atmospheric composition.]] | [[Image:Atmosphere composition diagram.jpg|thumb|right|350px|Diagram of chemical and transport processes related to atmospheric composition.]] | ||
The history of the Earth's atmosphere prior to one billion years ago is poorly understood, but the following presents a plausible sequence of events. This remains an active area of research. | The history of the Earth's atmosphere prior to one billion years ago is poorly understood, but the following presents a plausible sequence of events. This remains an active area of research. | ||
| − | The modern atmosphere is sometimes referred to as Earth's "third atmosphere", in order to distinguish the current [[chemical]] composition from two notably different previous compositions. The original atmosphere was primarily [[helium]] and [[hydrogen]] | + | The modern atmosphere is sometimes referred to as Earth's "third atmosphere", in order to distinguish the current [[chemical]] composition from two notably different previous compositions. The original atmosphere was primarily [[helium]] and [[hydrogen]]. [[Heat]] (from the still-molten crust, and the sun) dissipated this atmosphere. |
| − | About 3.5 billion years ago, the surface had cooled enough to form a [[crust]], still heavily populated with [[volcano]]es which released [[steam]], [[carbon dioxide]], and [[ammonia]]. This led to the "second atmosphere" | + | About 3.5 billion years ago, the surface had cooled enough to form a [[crust]], still heavily populated with [[volcano]]es which released [[steam]], [[carbon dioxide]], and [[ammonia]]. This led to the "second atmosphere", which was primarily carbon dioxide and [[water vapor]], with some [[nitrogen]] but virtually no [[oxygen]] (though very recent simulations run at the University of Waterloo and University of Colorado in 2005 suggested that it may have had up to 40% hydrogen [http://newsrelease.uwaterloo.ca/news.php?id=4348]). This second atmosphere had approximately 100 [[multiplication|times]] as much [[gas]] as the current atmosphere. It is generally believed that the [[greenhouse effect]], caused by high levels of carbon dioxide, kept the Earth from [[freezing]]. |
| − | During the next few | + | During the next few million years, water vapor [[condensation|condensed]] to form [[rain]] and [[ocean]]s, which began to dissolve carbon dioxide. Approximately 50% of the carbon dioxide would be absorbed into the oceans. One of the earliest types of bacteria were the [[cyanobacteria]]. Fossil evidence indicates that these bacteria existed approximately 3.3 billion years ago and were the first oxygen-producing evolving phototropic organisms. They were responsible for the initial conversion of the earth's atmosphere from an anoxic state to an oxic state (that is, from a state without oxygen to a state with oxygen). Being the first to carry out oxygenic photosynthesis, they were able to convert carbon dioxide into oxygen, playing a major role in oxygenating the atmosphere. |
| − | [[photosynthesis|Photosynthesizing]] plants would [[evolution|evolve]] and convert more carbon dioxide into oxygen. Over time, excess carbon became locked in [[fossil fuels]], [[sedimentary rock]]s (notably [[limestone]]), and [[animal shell]]s. As oxygen was released, it reacted with ammonia to create nitrogen; in addition, [[bacterium|bacteria]] would also convert ammonia into nitrogen. | + | [[photosynthesis|Photosynthesizing]] plants would later [[evolution|evolve]] and convert more carbon dioxide into oxygen. Over time, excess carbon became locked in [[fossil fuels]], [[sedimentary rock]]s (notably [[limestone]]), and [[animal shell]]s. As oxygen was released, it reacted with ammonia to create nitrogen; in addition, [[bacterium|bacteria]] would also convert ammonia into nitrogen. |
| − | As more plants appeared, the levels of oxygen increased significantly | + | As more plants appeared, the levels of oxygen increased significantly, while carbon dioxide levels dropped. At first the oxygen combined with various [[chemical element|element]]s (such as [[iron]]), but eventually oxygen accumulated in the atmosphere, resulting in [[mass extinction]]s and further evolution. With the appearance of an [[ozone layer]] (ozone is an [[allotrope]] of oxygen) [[life]]forms were better protected from [[ultraviolet radiation]]. This oxygen-nitrogen atmosphere is the "third atmosphere". |
== References == | == References == | ||
| − | *''[http://www.oma.be/BIRA-IASB/Public/Research/Thermo/Thermotxt.en.html The thermosphere: a part of the heterosphere]'', by J. Vercheval | + | *''[http://web.archive.org/web/20041030214502/http://www.oma.be/BIRA-IASB/Public/Research/Thermo/Thermotxt.en.html The thermosphere: a part of the heterosphere]'', by J. Vercheval. |
==See also== | ==See also== | ||
| − | |||
*[[Air glow]] | *[[Air glow]] | ||
*[[Atmospheric electricity]] | *[[Atmospheric electricity]] | ||
| + | *[[Atmospheric dispersion modeling]] | ||
| + | *[[Compressed air]] | ||
*[[Global warming]] | *[[Global warming]] | ||
*[[Greenhouse effect]] | *[[Greenhouse effect]] | ||
| Line 97: | Line 145: | ||
*[http://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html NASA's Earth Fact Sheet] | *[http://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html NASA's Earth Fact Sheet] | ||
*[http://atmospheres.agu.org/ American Geophysical Union: Atmospheric Sciences] | *[http://atmospheres.agu.org/ American Geophysical Union: Atmospheric Sciences] | ||
| + | *[http://www.srh.noaa.gov/srh/jetstream/atmos/layers.htm Layers of the Atmosphere] | ||
| + | *[http://amsglossary.allenpress.com/glossary The AMS Glossary of Meteorology] | ||
| + | {{earthsatmosphere}} | ||
| + | [[Category:Atmospheric sciences]] | ||
[[Category:Atmosphere]] | [[Category:Atmosphere]] | ||
[[Category:Environments]] | [[Category:Environments]] | ||
| − | [[bg: | + | [[ar:غلاف الأرض الجوي]] |
| + | [[bg:Атмосфера]] | ||
| + | [[bn:পৃথিবীর বায়ুমণ্ডল]] | ||
[[ca:Atmosfera terrestre]] | [[ca:Atmosfera terrestre]] | ||
| + | [[cs:Atmosféra]] | ||
[[da:Jordens atmosfære]] | [[da:Jordens atmosfære]] | ||
[[de:Erdatmosphäre]] | [[de:Erdatmosphäre]] | ||
| + | [[el:Ατμόσφαιρα]] | ||
[[es:Atmósfera terrestre]] | [[es:Atmósfera terrestre]] | ||
| − | [[eo:Atmosfero ( | + | [[eo:Atmosfero (tero)]] |
[[fr:Atmosphère de la Terre]] | [[fr:Atmosphère de la Terre]] | ||
| − | [[ko: | + | [[ko:지구 대기권]] |
[[id:Atmosfer]] | [[id:Atmosfer]] | ||
[[it:Atmosfera#L'atmosfera terrestre]] | [[it:Atmosfera#L'atmosfera terrestre]] | ||
| − | [[he: | + | [[he:אטמוספירת כדור הארץ]] |
| + | [[lt:Žemės atmosfera]] | ||
| + | [[mk:Атмосфера]] | ||
[[ms:Atmosfera]] | [[ms:Atmosfera]] | ||
[[nl:Aardatmosfeer]] | [[nl:Aardatmosfeer]] | ||
| − | [[ja: | + | [[ja:大気]] |
| − | |||
[[no:Jordens atmosfære]] | [[no:Jordens atmosfære]] | ||
| + | [[nn:Jordatmosfæren]] | ||
[[oc:Atmosfèra]] | [[oc:Atmosfèra]] | ||
| − | [[ru: | + | [[pt:Atmosfera]] |
| − | [[sk:Atmosféra]] | + | [[ru:Атмосфера Земли]] |
| − | [[sl: | + | [[sc:Atmosfera]] |
| + | [[simple:Atmosphere]] | ||
| + | [[sk:Atmosféra Zeme]] | ||
| + | [[sl:Ozračje]] | ||
| + | [[sr:Zemljina atmosfera]] | ||
[[fi:Ilmakehä]] | [[fi:Ilmakehä]] | ||
[[sv:Jordens atmosfär]] | [[sv:Jordens atmosfär]] | ||
| − | [[vi: | + | [[vi:Khí quyển Trái Đất]] |
| − | [[ | + | [[uk:Атмосфера]] |
| − | + | [[zh:地球大气层]] | |
| − | |||
| − | |||
| − | [[ | ||
Revision as of 22:55, 24 April 2006
- "Air" redirects here.
Earth's atmosphere is a layer of gases surrounding the planet Earth and retained by the Earth's gravity. It contains roughly 78% nitrogen and 21% oxygen, with trace amounts of other gases. This mixture of gases is commonly known as air. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation and reducing temperature extremes between day and night.
The atmosphere has no abrupt cut-off. It slowly becomes thinner and fades away into space. There is no definite boundary between the atmosphere and outer space. Three-quarters of the atmosphere's mass is within 11 km of the planetary surface. In the United States, persons who travel above an altitude of 50.0 miles (80.5 km) are designated as astronauts. An altitude of 120 km (75 mi or 400,000 ft) marks the boundary where atmospheric effects become noticeable during re-entry. The Karman line, at 100 km (62 mi), is also frequently used as the boundary between atmosphere and space.
Temperature and the atmospheric layers
The temperature of the Earth's atmosphere varies with altitude; the mathematical relationship between temperature and altitude varies between the different atmospheric layers:
- troposphere: From the Greek word "tropos" meaning to turn or mix. The troposphere is the lowest layer of the atmosphere starting at the surface going up to between 7 km at the poles and 17 km at the equator with some variation due to weather factors. The troposphere has a great deal of vertical mixing due to solar heating at the surface. This heating warms air masses, which then rise to release latent heat as sensible heat that further buoys the air mass. This process continues until all water vapor is removed. In the troposphere, on average, temperature decreases with height due to expansive cooling.
- stratosphere: from that 7–17 km range to about 50 km, temperature increasing with height.
- mesosphere: from about 50 km to the range of 80 km to 85 km, temperature decreasing with height.
- thermosphere: from 80–85 km to 640+ km, temperature increasing with height.
The boundaries between these regions are named the tropopause, stratopause, and mesopause.
The average temperature of the atmosphere at the surface of earth is 14 °C.
Various atmospheric regions
Atmospheric regions are also named in other ways:
- ionosphere – the region containing ions: approximately the mesosphere and thermosphere up to 550 km.
- exosphere – above the ionosphere, where the atmosphere thins out into space. This is the last major atmosphere.
- magnetosphere – the region where the Earth's magnetic field interacts with the solar wind from the Sun. It extends for tens of thousands of kilometers, with a long tail away from the Sun.
- ozone layer – or ozonosphere, approximately 10 - 50 km, where stratospheric ozone is found. Note that even within this region, ozone is a minor constituent by volume.
- upper atmosphere – the region of the atmosphere above the mesopause.
- Van Allen radiation belts – regions where particles from the Sun become concentrated.
Pressure
- Barometric Formula: (used for airplane flight) barometric formula
- Main article: Atmospheric pressure
- Nasa mathematical model: NRLMSISE-00
Atmospheric pressure is a direct result of the weight of the air. This means that air pressure varies with location and time, because the amount (and weight) of air above the earth varies with location and time. Atmospheric pressure drops by ~50% at an altitude of about 5 km (equivalently, about 50% of the total atmospheric mass is within the lowest 5 km). The average atmospheric pressure, at sea level, is about 101.3 kilopascals (about 14.7 pounds per square inch).
Thickness of the atmosphere
Although the atmosphere exists at heights of 1000 km and more, it is so thin as to be considered nonexistant.
- 57.8% of the atmosphere is below the summit of Mount Everest.
- 72% of the atmosphere is below the common cruising altitude of commercial airliners (about 10000 m or 32800 ft).
- 99.99999% of the atmosphere is below the highest X-15 plane flight on August 22, 1963, which reached an altitude of 354,300 ft or 108 km.
Therefore, most of the atmosphere (99.9999%) is below 100 km, although in the rarified region above this there are auroras and other atmospheric effects.
Composition
| ppmv: parts per million by volume | |
| Gas | Volume |
|---|---|
| Nitrogen (N2) | 780,840 ppmv (78.084%) |
| Oxygen (O2) | 209,460 ppmv (20.946%) |
| Argon (Ar) | 9,340 ppmv (0.9340%) |
| Carbon dioxide (CO2) | 350 ppmv |
| Neon (Ne) | 18.18 ppmv |
| Helium (He) | 5.24 ppmv |
| Methane (CH4) | 1.745 ppmv |
| Krypton (Kr) | 1.14 ppmv |
| Hydrogen (H2) | 0.55 ppmv |
| Not included in above dry atmosphere: | |
| Water vapor (highly variable) | typically 1% |
Source for figures above: NASA. Carbon dioxide and methane updated (to 1998) by IPCC TAR table 6.1 [1]. The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2 by about 7 ppmv.
Minor components of air not listed above include:
| Gas | Volume |
|---|---|
| nitrous oxide | 0.5 ppmv |
| xenon | 0.09 ppmv |
| ozone | 0.0 to 0.07 ppmv |
| nitrogen dioxide | 0.02 ppmv |
| iodine | 0.01 ppmv |
| carbon monoxide | trace |
| ammonia | trace |
- The mean molecular mass of air is 28.97 g/mol.
Heterosphere
Below the turbopause at an altitude of about 100 km, the Earth's atmosphere has a more-or-less uniform composition (apart from water vapor) as described above; this constitutes the homosphere.[2] However, above about 100 km, the Earth's atmosphere begins to have a composition which varies with altitude. This is essentially because, in the absence of mixing, the density of a gas falls off exponentially with increasing altitude, but at a rate which depends on the molecular mass. Thus higher mass constituents, such as oxygen and nitrogen, fall off more quickly than lighter constituents such as helium, molecular hydrogen, and atomic hydrogen. Thus there is a layer, called the heterosphere, in which the earth's atmosphere has varying composition. As the altitude increases, the atmosphere is dominated successively by helium, molecular hydrogen, and atomic hydrogen. The precise altitude of the heterosphere and the layers it contains varies significantly with temperature.[3]
Density and mass
- Main article: Density of air
The density of air at sea level is about 1.2 kg/m3. Natural variations of the barometric pressure occur at any one altitude as a consequence of weather. This variation is relatively small for inhabited altitudes but much more pronounced in the outer atmosphere and space due to variable solar radiation.
The atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the barometric formula. More sophisticated models are used by meteorologists and space agencies to predict weather and orbital decay of satellites.
The average mass of the atmosphere is about 5,000 trillion metric tons. According to the National Center for Atmospheric Research, "The total mean mass of the atmosphere is 5.1480 x 1018 kg with an annual range due to water vapor of 1.2 or 1.5 x 1015 kg depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27 x 1016 kg and the dry air mass as 5.1352 ±0.0003 x 1018 kg."
The above composition percentages are done by volume. Assuming that the gases act like ideal gases, we can add the percentages p multiplied by their molar masses m, to get a total t = sum (p·m). Any element's percent by mass is then p·m/t. When we do this to the above percentages, we get that, by mass, the composition of the atmosphere is 75.523% nitrogen, 23.133% oxygen, 1.288% argon, 0.053% carbon dioxide, 0.001267% neon, 0.00029% methane, 0.00033% krypton, 0.000724% helium, and 0.0000038 % hydrogen.
The evolution of the Earth's atmosphere
The history of the Earth's atmosphere prior to one billion years ago is poorly understood, but the following presents a plausible sequence of events. This remains an active area of research.
The modern atmosphere is sometimes referred to as Earth's "third atmosphere", in order to distinguish the current chemical composition from two notably different previous compositions. The original atmosphere was primarily helium and hydrogen. Heat (from the still-molten crust, and the sun) dissipated this atmosphere.
About 3.5 billion years ago, the surface had cooled enough to form a crust, still heavily populated with volcanoes which released steam, carbon dioxide, and ammonia. This led to the "second atmosphere", which was primarily carbon dioxide and water vapor, with some nitrogen but virtually no oxygen (though very recent simulations run at the University of Waterloo and University of Colorado in 2005 suggested that it may have had up to 40% hydrogen [4]). This second atmosphere had approximately 100 times as much gas as the current atmosphere. It is generally believed that the greenhouse effect, caused by high levels of carbon dioxide, kept the Earth from freezing.
During the next few million years, water vapor condensed to form rain and oceans, which began to dissolve carbon dioxide. Approximately 50% of the carbon dioxide would be absorbed into the oceans. One of the earliest types of bacteria were the cyanobacteria. Fossil evidence indicates that these bacteria existed approximately 3.3 billion years ago and were the first oxygen-producing evolving phototropic organisms. They were responsible for the initial conversion of the earth's atmosphere from an anoxic state to an oxic state (that is, from a state without oxygen to a state with oxygen). Being the first to carry out oxygenic photosynthesis, they were able to convert carbon dioxide into oxygen, playing a major role in oxygenating the atmosphere.
Photosynthesizing plants would later evolve and convert more carbon dioxide into oxygen. Over time, excess carbon became locked in fossil fuels, sedimentary rocks (notably limestone), and animal shells. As oxygen was released, it reacted with ammonia to create nitrogen; in addition, bacteria would also convert ammonia into nitrogen.
As more plants appeared, the levels of oxygen increased significantly, while carbon dioxide levels dropped. At first the oxygen combined with various elements (such as iron), but eventually oxygen accumulated in the atmosphere, resulting in mass extinctions and further evolution. With the appearance of an ozone layer (ozone is an allotrope of oxygen) lifeforms were better protected from ultraviolet radiation. This oxygen-nitrogen atmosphere is the "third atmosphere".
ReferencesISBN links support NWE through referral fees
- The thermosphere: a part of the heterosphere, by J. Vercheval.
See also
- Air glow
- Atmospheric electricity
- Atmospheric dispersion modeling
- Compressed air
- Global warming
- Greenhouse effect
- Historical temperature record
- Intergovernmental Panel on Climate Change (IPCC)
External links
- NASA atmosphere models
- NASA's Earth Fact Sheet
- American Geophysical Union: Atmospheric Sciences
- Layers of the Atmosphere
- The AMS Glossary of Meteorology
|
Troposphere | Stratosphere | Mesosphere | Thermosphere | Exosphere |
|
Tropopause | Stratopause | Mesopause | Exobase |
|
Ozone layer | Turbopause | Ionosphere |
ar:غلاف الأرض الجوي bg:Атмосфера bn:পৃথিবীর বায়ুমণ্ডল ca:Atmosfera terrestre cs:Atmosféra da:Jordens atmosfære de:Erdatmosphäre el:Ατμόσφαιρα es:Atmósfera terrestre eo:Atmosfero (tero) fr:Atmosphère de la Terre ko:지구 대기권 id:Atmosfer it:Atmosfera#L'atmosfera terrestre he:אטמוספירת כדור הארץ lt:Žemės atmosfera mk:Атмосфера ms:Atmosfera nl:Aardatmosfeer ja:大気 no:Jordens atmosfære nn:Jordatmosfæren oc:Atmosfèra pt:Atmosfera ru:Атмосфера Земли sc:Atmosfera simple:Atmosphere sk:Atmosféra Zeme sl:Ozračje sr:Zemljina atmosfera fi:Ilmakehä sv:Jordens atmosfär vi:Khí quyển Trái Đất uk:Атмосфера zh:地球大气层