Carbohydrate
Carbohydrates are a class of biological molecules that contian primarily carbon atoms flanked by hydrogen atoms , and hydroxyl groups (H-C-OH). Carbohydrates have two major biochemical roles. For one, they act as a source of energy that can be released in a form usable by bodily tissues. Secondly, they serve as carbon skeletons that can be rearranged to form other molecules necessary for biological structures and functions.
Some carbohydrates are small with molecular weights of less than 100 whereas others are true macromolecules with molecular weights in the hundreds of thousands. The four categories of carbohydrates are classified by thier number of sugar units:
- Monosaccharides (mono- "one," saccharide- "sugar") are the monomers out of which larger carbohydrates are constructed. Monosaccharides such as glucose, ribose and fructose are simple sugars
- Disaccharides (di-"two") such as sucrose and lactose are two monosaccharides linked together by covalent bonds.
- Oligosaccharides (oligo- "several") are made up of from 3 to 20 monosaccharides.
- Polysaccharides (poly- "many") are large polymers composed of hundreds or thousands of monosaccharides. Starch, glycogen, and cellulose are polysaccharides.
The general chemical formula for carbohydrates, C(H2O), gives the relative proportions of carbon, hydrogen, and oxygen in a monosaccharide (the proportion of these atoms are 1:2:1). This formula is characteristic of sugars and gave rise to the terms carbohydrate because compounds of this sort were originally thought to be "hydrates of carbon." This term persists even though a carbohydrate is definitely not a hydrated carbon atom. In disaccharides, oligosaccharides, and polysaccharidesn the molar proportions deviate slightly from the general formula because two hydrogens and one oxygen are lost during each of the condensation reactions that forms them. These carbohydrates have the more general formula Cn(H2O)m.
Monosaccharides
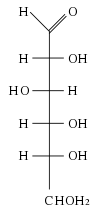
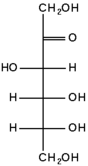
The repeating units of polysaccharides are simple sugars called monosaccharides. There are two categories of sugars: aldosugars, with a terminal carbonyl group, and ketosugars, with an internal carbonyl group typically on the second carbon atom. Within these two groups, sugars are named according to the number of carbon atoms they contain. Most sugars have between three and seven carbon atoms are therefore termed triose (three carbons), tetrose (four carbons), pentose (five carbons), hexose (six carbons), or heptose (seven carbons). Glucose is an aldohexose, fructose is a ketohexose, and ribose is an aldopentose. Each carbon atom that supports a hydroxyl group (except for the first and last) is optically active, allowing a number of different carbohydrates with the same basic structure. For instance, galactose is an aldohexose but has different properties from glucose because the atoms are arranged differently.
The single most common monosaccharide is the aldohexose D-glucose, represented by the formula C6H12O6. The carbons of glucose are numbered beginning with the more oxidized end of the molecule, the carbonyl group. The figure to the left depicts glucose as a linear molecule. In the cell however, glucose exists in dynamic equilibrium between the linear and ring configurations. The ring form is the predominant structure becuase it is energectially more stable. This form results from the addition of the hydroxyl (OH) group on carbon atom 5 across the carbonyl group of carbon atom 1.
A more satisfactory representation of glucose is shown in the Haworth projection. The Haworth projection is preferred because it indicates both the ring form and the spatial relationship between the carbon atoms. The tetrahedral nature of each carbon atom in the chain actually favors the ring formation of glucose. The formation of the ring structuregenerates two alternative forms glucose based on the spatla orientation of the hydroxyl group on carbon atom 1. These alternative forms of glucose are designated α and β. As shown in the figure, α-D-glucose has the hydroxyl group on carbon atom 1 pointing downward whereas β-D-glucose has the hydroxyl group on carbon atom 1 pointing upward. Starch and glycogen are composed of α-D-glucose monomers whereas cellulose is coposed of β-D-glucose monomers. Glucose interconverts between α-ring, β-ring, and straight-chain forms at dynamic equilibrium.
Disaccharides
Disaccharides are composed of two monosaccharide units bound together by a covalent glycosidic bond. The binding between the two sugars results in the loss of a hydrogen atom (H) from one molecule and a hydroxyl group (OH) from the other.
The most common disaccharides are sucrose (cane or beet sugar - made from one glucose and one fructose), lactose (milk sugar - made from one glucose and one galactose) and maltose (made of two glucoses). The formula of these disaccharides is C12H22O11.
Oligosaccharides and polysaccharides
- Main articles: Oligosaccharide and Polysaccharide
Oligosaccharides and polysaccharides are composed of longer chains of monosaccharide units bound together by glycosidic bonds. The distinction between the two is based upon the number of monosaccharide units present in the chain. Oligosaccharides typically contain between three and nine monosaccharide units, and polysaccharides contain greater than ten monosaccharide units. Definitions of how large a carbohydrate must be to fall into each category vary however.
Oligosaccharides are found as a common form of protein posttranslational modification. Polysaccharides represent an important class of biological polymer. Examples include starch, cellulose, chitin and glycogen.
Nutrition

Strictly speaking, carbohydrates are not necessary for human nutrition because proteins can be converted to carbohydrates. The traditional diet of some cultures consists of very little carbohydrate, and these people remain relatively healthy. However, carbohydrates require less water to digest than proteins or fats and are the most common source of energy. Proteins and fat are vital building components for body tissue and cells, and thus it could be considered advisable not to deplete such resources by necessitating their use in energy production.
Based on evidence for risk of heart disease and obesity, the Institute of Medicine recommends that American and Canadian adults get between 40-65% of dietary energy from carbohydrates.[1] The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55-75% of total energy from carbohydrates.[2]
Very low carbohydrate diets can slow down brain and neural function because the nervous system especially relies on glucose.
Some problems have been cited for the long term effects of a no-carbohydrate diet for some individuals. Athletes, for instance, or those that participate in high intensity activities, will have a considerable reduction in performance, due to having little or no glycogen supplies stored in muscle tissue. Additionally, nephrotoxicity may occur, particularly in persons that are not very well hydrated.
Foods high in carbohydrates
Breads, pastas, potatos, bran and cereals are all high in carbohydrates.
Classification
Dietitians and nutritionists commonly classify carbohydrates as simple (monosaccharides and disaccharides) or complex (oligosaccharides and polysaccharides), depending on their chemical structure. The term complex carbohydrate was first used in the Senate Select Committee publication Dietary Goals for the United States (1977), where it denoted "fruit, vegetables and whole-grains".[3] Dietary guidelines generally recommend that complex carbohydrates and nutrient-rich simple carbohydrates such as fruit and dairy products should make up the bulk of carbohydrate consumption. The USDA's Dietary Guidelines for Americans 2005 dispenses with the simple/complex distinction, instead recommending fiber-rich foods and whole grains.[4]
The glycemic index and glycemic load systems are popular alternative classification methods which rank carbohydrates based on their effect on blood glucose levels.
Catabolism
There are two major metabolic pathways of carbohydrate catabolism:
ReferencesISBN links support NWE through referral fees
- ↑ Food and Nutrition Board (2002/2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. Page 769. ISBN 0-309-08537-3
- ↑ Joint WHO/FAO expert consultation (2003). Diet, Nutrition and the Prevention of Chronic Diseases (PDF). Geneva: World Health Organization. Pages 55-56. ISBN 92-4-120916-X
- ↑ Joint WHO/FAO expert consultation (1998), Carbohydrates in human nutrition, chapter 1. ISBN 92-5-104114-8.
- ↑ DHHS and USDA, Dietary Guidelines for Americans 2005, Chapter 7 Carbohydrates
External links
- IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature
- Carbohydrates Information
- Carbohydrates detailed
- Carbohydrates Overview
- Carbohydrates and Glycosylation - The Virtual Library of Biochemistry and Cell Biology
- Consortium for Functional Glycomics
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.