Difference between revisions of "Carbohydrate" - New World Encyclopedia
| Line 45: | Line 45: | ||
Starch occurs as both unbranched [[amylose]] and branched [[amylopectin]]. Like glycogen, amylopectin has α-1,6 branches, but these occur less frequently along the helical backbone (once every 12 to 25 glucose units), producing longer side chains (lengths of 20 to 25 glucose units). Starch readily binds water, and when that water is removed, polysaccharide chains aggregate, forming [[hydrogen bond]]s. This bonding is what causes bread to become hard and stale. The addition of water and gentle heat softens the bread by separating the polysaccharide chains. Since branching limits the number of hydrogen bonds that can form betweeen molecules, solid deposits of the highly-branched glycogen are more compact than those of starch. Starch deposits are generall about 10-30% amylose and 70-90% amylopectin. | Starch occurs as both unbranched [[amylose]] and branched [[amylopectin]]. Like glycogen, amylopectin has α-1,6 branches, but these occur less frequently along the helical backbone (once every 12 to 25 glucose units), producing longer side chains (lengths of 20 to 25 glucose units). Starch readily binds water, and when that water is removed, polysaccharide chains aggregate, forming [[hydrogen bond]]s. This bonding is what causes bread to become hard and stale. The addition of water and gentle heat softens the bread by separating the polysaccharide chains. Since branching limits the number of hydrogen bonds that can form betweeen molecules, solid deposits of the highly-branched glycogen are more compact than those of starch. Starch deposits are generall about 10-30% amylose and 70-90% amylopectin. | ||
| + | |||
| + | Cellulose is the main component of plant [[cell wall]]s and is by far the most abundant [[organic]] (carbon-containing) compound on earth. Like starch and glycogen, cellulose is also a polymer of glucose, but the repeating monosaccharidee unit is β-glucose and the linkage is therefore β-1,4. Because of the stability of its β-glycosidic linkages, cellulose is an excellent structural material that can withstand harsh environmental conditions. Mammals do not have an enzyme that can hydrolyze a β-1,4 bond, therefore, mammals cannot use cellulose as a food. For this reason, humans can digest potatoes (starch) but not grass (cellulose). Animals such as cow and sheep that eat grass cannot cleave β-glycosidic bonds either, but rather depend on the bactera and protoza in their [[rumen]] (part of their compound stomach) to do this. These microorganisms digest cellulose and create end-products in the form that the animal can use. The rigid linear rods that cellulose forms aggrgates laterally into [[microfibrils]]. Microfibrils are about 25 nm in diameter and are made up of about 2000 cellulose chains. The cells walls of plants and fungi consist of cellulose microfibrils embedded in a [[noncellulosic matrix]] containg a variable mixture of several other polymers. | ||
==Nutrition== | ==Nutrition== | ||
Revision as of 06:55, 4 June 2006
Carbohydrates are a class of biological molecules that contian primarily carbon atoms flanked by hydrogen atoms , and hydroxyl groups (H-C-OH). Carbohydrates have two major biochemical roles. For one, they act as a source of energy that can be released in a form usable by bodily tissues. Secondly, they serve as carbon skeletons that can be rearranged to form other molecules necessary for biological structures and functions.
Some carbohydrates are small with molecular weights of less than 100 whereas others are true macromolecules with molecular weights in the hundreds of thousands. The four categories of carbohydrates are classified by thier number of sugar units:
- Monosaccharides (mono- "one," saccharide- "sugar") are the monomers out of which larger carbohydrates are constructed. Monosaccharides such as glucose, ribose and fructose are simple sugars
- Disaccharides (di-"two") such as sucrose and lactose are two monosaccharides linked together by covalent bonds.
- Oligosaccharides (oligo- "several") are made up of from 3 to 20 monosaccharides.
- Polysaccharides (poly- "many") are large polymers composed of hundreds or thousands of monosaccharides. Starch, glycogen, and cellulose are polysaccharides.
The general chemical formula for carbohydrates, C(H2O), gives the relative proportions of carbon, hydrogen, and oxygen in a monosaccharide (the proportion of these atoms are 1:2:1). This formula is characteristic of sugars and gave rise to the terms carbohydrate because compounds of this sort were originally thought to be "hydrates of carbon." This term persists even though a carbohydrate is definitely not a hydrated carbon atom. In disaccharides, oligosaccharides, and polysaccharidesn the molar proportions deviate slightly from the general formula because two hydrogens and one oxygen are lost during each of the condensation reactions that forms them. These carbohydrates have the more general formula Cn(H2O)m.
Monosaccharides
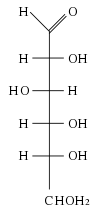
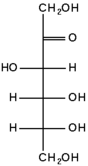
The repeating units of polysaccharides are simple sugars called monosaccharides. There are two categories of sugars: aldosugars, with a terminal carbonyl group, and ketosugars, with an internal carbonyl group typically on the second carbon atom. Within these two groups, sugars are named according to the number of carbon atoms they contain. Most sugars have between three and seven carbon atoms are therefore termed triose (three carbons), tetrose (four carbons), pentose (five carbons), hexose (six carbons), or heptose (seven carbons). Glucose is an aldohexose, fructose is a ketohexose, and ribose is an aldopentose. Each carbon atom that supports a hydroxyl group (except for the first and last) is optically active, allowing a number of different carbohydrates with the same basic structure. For instance, galactose is an aldohexose but has different properties from glucose because the atoms are arranged differently.
The single most common monosaccharide is the aldohexose D-glucose, represented by the formula C6H12O6. The carbons of glucose are numbered beginning with the more oxidized end of the molecule, the carbonyl group. The figure to the left depicts glucose as a linear molecule. In the cell however, glucose exists in dynamic equilibrium between the linear and ring configurations. The ring form is the predominant structure becuase it is energectially more stable. This form results from the addition of the hydroxyl (OH) group on carbon atom 5 across the carbonyl group of carbon atom 1.
A more satisfactory representation of glucose is shown in the Haworth projection. The Haworth projection is preferred because it indicates both the ring form and the spatial relationship between the carbon atoms. The tetrahedral nature of each carbon atom in the chain actually favors the ring formation of glucose. The formation of the ring structuregenerates two alternative forms glucose based on the spatla orientation of the hydroxyl group on carbon atom 1. These alternative forms of glucose are designated α and β. As shown in the figure, α-D-glucose has the hydroxyl group on carbon atom 1 pointing downward whereas β-D-glucose has the hydroxyl group on carbon atom 1 pointing upward. Starch and glycogen are composed of α-D-glucose monomers whereas cellulose is coposed of β-D-glucose monomers. Glucose interconverts between α-ring, β-ring, and straight-chain forms at dynamic equilibrium.
Disaccharides and Oligosaccharides
In addition to the free monosaccharide, glucose also occurs in disaccharides, which consist of two monosaccharide units linked covalently. Each disaccharide is formed by a condensation reaction in which there is a loss of a hydrogen (H) from one molecule and a hydroxyl group (OH) from the other. Glycosidic bonds form between carbon 1 of the first glucose molecule and carbon 4 of the second glucose molecule. The resulting glycosidic bond is the characteristic linkage between sugars. Three common dissaccharides are shown in the figure. Maltose (malt sugar) is made up of two glucose units linked togeether. Lactose (milk sugar) consists of a glucose linked to a galactose. Sucrose (common table sugar) has a glucose linked to a fructose.
Although the disaccharide maltose contains two glucose molecules, it is not the only disaccharide that can be made from two glucoses. When glucose molecules form a glycosidic bond, the linkage will be one of two types, α or β, deopending on whether the molecule that bonds its carbon 1 is an α-glucose or β-glucose. An α-linkage with carbon 4 of a second glucose molecule results in maltose, whereas a β-linkage results in cellobiose. Although maltose and cellobiose are disaccharide isomers, both having the formula C12H22O11, they are different compounds with different properties. For example, maltose can be hydrolyzed to its monosaccharides in the human body where as cellobiose cannot. Some organisms have the capacity to bread down cellobiose.
Oligosaccharides typically consist of three to nine monosaccharide units bound by glycosidic linkages. Often, oligosaccharides have additional functional groups which give them special properties. Many oligosaccharides are covalently bonded to proteins and lipids on the outer cell surface where they play important roles in cellular recognition of extracellular signal molecules and of other cells. The human blood groups (ABO) obtain their specificity from oligosaccharide polymers.
Polysaccharides
- Main articles: Oligosaccharide and Polysaccharide
Polysaccharides are giant polymers of monosaccharides linked by glycosidic bonds. Polysacchardies are not informational molecules. The major polysacchardides in higher organisms are the storage polysaccharides starch (in plant cells) and glycogen (in animal cells), in addition to the structual polysaccharide cellulose (in plant cells). Each of these polymers contain the six-carbon sugar glucose as its single repeating unit, but they differ in the type of bond between glucose units and the presence and extent of side branches on the chains.
- Starch is a polysaccharide of glucose with α-1,4 glycosidic linkages.
- Glycogen is a highly branched polysaccharde of glucose with α-glycosidic linkages. α-1,6 glycosidic linkages produce branching at carbon 6.
- Cellulose is an unbranched polysaccharide of glucose with β-1,4 glycosidic linkages that are chemically very stable.
Glycogen is highly branched with α-1, 6 linkages occuring every 8 to 10 glucose units along the backbone and giving rise to short side chains of about 8 to 12 glucose units. Glycogen is stored mainly in the liver, and in muscle tissue. In the liver, glycogen is readily hydrolyzed to glucose monomers which are used to maintain blood sugar levels. In muscle, glucose monomers of glycogen are further degraded to liberate their stored energy for generation of the ATP needed for muscle contraction. The reason that glucose must be stored as the polymer glycogen is that 1000 glucose molecules would exert 1,000 times the osmotic pressure (causing water to ener the cells) of a single glycogen molecule. Without polysaccharides, organisms would expend a lot of time and energy expelling excess water.
Starch occurs as both unbranched amylose and branched amylopectin. Like glycogen, amylopectin has α-1,6 branches, but these occur less frequently along the helical backbone (once every 12 to 25 glucose units), producing longer side chains (lengths of 20 to 25 glucose units). Starch readily binds water, and when that water is removed, polysaccharide chains aggregate, forming hydrogen bonds. This bonding is what causes bread to become hard and stale. The addition of water and gentle heat softens the bread by separating the polysaccharide chains. Since branching limits the number of hydrogen bonds that can form betweeen molecules, solid deposits of the highly-branched glycogen are more compact than those of starch. Starch deposits are generall about 10-30% amylose and 70-90% amylopectin.
Cellulose is the main component of plant cell walls and is by far the most abundant organic (carbon-containing) compound on earth. Like starch and glycogen, cellulose is also a polymer of glucose, but the repeating monosaccharidee unit is β-glucose and the linkage is therefore β-1,4. Because of the stability of its β-glycosidic linkages, cellulose is an excellent structural material that can withstand harsh environmental conditions. Mammals do not have an enzyme that can hydrolyze a β-1,4 bond, therefore, mammals cannot use cellulose as a food. For this reason, humans can digest potatoes (starch) but not grass (cellulose). Animals such as cow and sheep that eat grass cannot cleave β-glycosidic bonds either, but rather depend on the bactera and protoza in their rumen (part of their compound stomach) to do this. These microorganisms digest cellulose and create end-products in the form that the animal can use. The rigid linear rods that cellulose forms aggrgates laterally into microfibrils. Microfibrils are about 25 nm in diameter and are made up of about 2000 cellulose chains. The cells walls of plants and fungi consist of cellulose microfibrils embedded in a noncellulosic matrix containg a variable mixture of several other polymers.
Nutrition

Strictly speaking, carbohydrates are not necessary for human nutrition because proteins can be converted to carbohydrates. The traditional diet of some cultures consists of very little carbohydrate, and these people remain relatively healthy. However, carbohydrates require less water to digest than proteins or fats and are the most common source of energy. Proteins and fat are vital building components for body tissue and cells, and thus it could be considered advisable not to deplete such resources by necessitating their use in energy production.
Based on evidence for risk of heart disease and obesity, the Institute of Medicine recommends that American and Canadian adults get between 40-65% of dietary energy from carbohydrates.[1] The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55-75% of total energy from carbohydrates.[2]
Very low carbohydrate diets can slow down brain and neural function because the nervous system especially relies on glucose.
Some problems have been cited for the long term effects of a no-carbohydrate diet for some individuals. Athletes, for instance, or those that participate in high intensity activities, will have a considerable reduction in performance, due to having little or no glycogen supplies stored in muscle tissue. Additionally, nephrotoxicity may occur, particularly in persons that are not very well hydrated.
Foods high in carbohydrates
Breads, pastas, potatos, bran and cereals are all high in carbohydrates.
Classification
Dietitians and nutritionists commonly classify carbohydrates as simple (monosaccharides and disaccharides) or complex (oligosaccharides and polysaccharides), depending on their chemical structure. The term complex carbohydrate was first used in the Senate Select Committee publication Dietary Goals for the United States (1977), where it denoted "fruit, vegetables and whole-grains".[3] Dietary guidelines generally recommend that complex carbohydrates and nutrient-rich simple carbohydrates such as fruit and dairy products should make up the bulk of carbohydrate consumption. The USDA's Dietary Guidelines for Americans 2005 dispenses with the simple/complex distinction, instead recommending fiber-rich foods and whole grains.[4]
The glycemic index and glycemic load systems are popular alternative classification methods which rank carbohydrates based on their effect on blood glucose levels.
Catabolism
There are two major metabolic pathways of carbohydrate catabolism:
ReferencesISBN links support NWE through referral fees
- ↑ Food and Nutrition Board (2002/2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. Page 769. ISBN 0-309-08537-3
- ↑ Joint WHO/FAO expert consultation (2003). Diet, Nutrition and the Prevention of Chronic Diseases (PDF). Geneva: World Health Organization. Pages 55-56. ISBN 92-4-120916-X
- ↑ Joint WHO/FAO expert consultation (1998), Carbohydrates in human nutrition, chapter 1. ISBN 92-5-104114-8.
- ↑ DHHS and USDA, Dietary Guidelines for Americans 2005, Chapter 7 Carbohydrates
External links
- IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature
- Carbohydrates Information
- Carbohydrates detailed
- Carbohydrates Overview
- Carbohydrates and Glycosylation - The Virtual Library of Biochemistry and Cell Biology
- Consortium for Functional Glycomics
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.