Butyric acid
| Butyric acid | |
|---|---|
| |
| |
| IUPAC name | butyric acid |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| MeSH | |
| SMILES | CCCC(=O)O |
| Properties | |
| Molecular formula | C4H8O2 |
| Molar mass | 88.1051 |
| Melting point |
-7.9 °C (265.1 K) |
| Boiling point |
163.5 °C (436.5 K) |
| Hazards | |
| R-phrases | 34 |
| S-phrases | 26 36 45 |
| Flash point | 72 °C |
| RTECS number | ES5425000 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
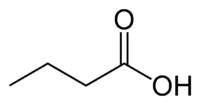
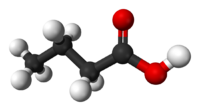
Butyric acid, also known as n-Butanoic acid (in the IUPAC[1] system) or normal butyric acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. It is classified as a short-chain fatty acid. It has an unpleasant odor and acrid taste, but a sweetish aftertaste (similar to ether). It is notably found in rancid butter, parmesan cheese, and vomit. Its name is derived from the Greek word βουτυρος, which means "butter." Certain esters of butyric acid have a pleasant taste or smell, and they are used as additives in foods and perfumes.
Occurrence and preparation
Normal butyric acid occurs in the form of esters in animal fats and plant oils. The glyceride of butyric acid makes up 3-4 percent of butter. When butter goes rancid, butyric acid is liberated from the glyceride by hydrolysis, leading to the unpleasant odor.
Normal butyric acid or fermentation butyric acid is also found as a hexyl ester in the oil of Heracleum giganteum (cow parsnip) and as an octyl ester in parsnip (Pastinaca sativa); it has also been noticed in the fluids of the flesh and in perspiration.
It is ordinarily prepared by the fermentation of sugar or starch, brought about by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed in the process. The butyric fermentation of starch is aided by the direct addition of Bacillus subtilis.
Notable characteristics
Butyric acid is an oily, colorless liquid that solidifies at -8 °C and boils at 164 °C. It is easily soluble in water, ethanol, and ether, and is thrown out of its aqueous solution by the addition of calcium chloride. Potassium dichromate and sulfuric acid (or sulphuric acid) oxidize it to carbon dioxide and acetic acid. Alkaline potassium permanganate oxidizes it to carbon dioxide. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot water than in cold.
Butyric acid can be detected by mammals with good scent detection abilities (such as dogs) at 10 ppb, while humans can detect it in concentrations above 10 ppm.
An isomer, called isobutyric acid, has the same chemical formula (C4H8 O2) but a different structure. It has similar chemical properties but different physical properties.
Applications
Butyric acid is used in the preparation of various butyrate esters. Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aromas or tastes. As a consequence, they find use as food and perfume additives. They are also used in organic laboratory courses, to teach the Fisher esterification reaction.
Butyrate fermentation
Butyrate is produced as the end-product of a fermentation process solely performed by obligate anaerobic bacteria. Kombucha tea includes butyric acid as a result of fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861. Examples of butyrate producing species :
- Clostridium butyricum
- Clostridium kluyveri
- Clostridium pasteurianum
- Fusobacterium nucleatum
- Butyrivibrio fibrisolvens
- Eubacterium limosum
The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate-ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed in the process and exit the cell. Then:
| Action | Responsible enzyme |
| Acetyl coenzyme A converts into acetoacetyl coenzyme A | acetyl-CoA-acetyl transferase |
| Acetoacetyl coenzyme A converts into β-hydroxybutyryl CoA | β-hydroxybutyryl-CoA dehydrogenase |
| β-hydroxybutyryl CoA converts into crotonyl CoA | crotonase |
| Crotonyl CoA converts into butyryl CoA (CH3CH2CH2C=O-CoA) | butyryl CoA dehydrogenase |
| A phosphate group replaces CoA to form butyryl phosphate | phosphobutyrylase |
| The phosphate group joins ADP to form ATP and butyrate | butyrate kinase |
ATP is produced, as can be seen, in the last step of the fermentation. 3 ATPs are produced for each glucose molecule, a relatively high yield. The balanced equation for this fermentation is:
C6H12O6 → C4H8O2 + 2CO2 + 2H2
Acetone and butanol fermentation
Several species form acetone and butanol in an alternative pathway which starts as butyrate fermentation. Some of these species are:
- Clostridium acetobutylicum: the most prominent acetone and butanol producer, used also industrially
- Clostridium beijerinckii
- Clostridium tetanomorphum
- Clostridium aurantibutyricum
These bacteria begin with butyrate fermentation as described above, but, when the pH drops below 5, they switch into butanol and acetone production in order to prevent further lowering of the pH. Two molecules of butanol are formed for each molecule of acetone.
The change in the pathway occurs after acetoacetyl CoA formation. This intermediate then takes two possible pathways:
- Acetoacetyl CoA → acetoacetate → acetone, or
- Acetoacetyl CoA → butyryl CoA → butyraldehyde → butanol.
Butyric acid function/activity
Highly fermentable fibers like oat bran, pectin, and guar are transformed by colonic bacteria into short chain fatty acids including butyrate.
Butyrate has diverse and apparently paradoxical effects on cellular proliferation, apoptosis and differentiation that may be either pro-neoplastic or anti-neoplastic, depending upon factors such as the level of exposure, availability of other metabolic substrate and the intracellular milieu. Butyrate is thought by some to be protective against colon cancer. However, not all studies support a chemopreventive effect for butyrate and the lack of agreement (particularly between in vivo and in vitro studies) on butyrate and colon cancer has been termed the "butyrate paradox." There are a number of reasons for this discrepant effect including differences between the in vitro and in vivo environments, the timing of butyrate administration, the amount of butyrate administered, the source of butyrate (usually dietary fiber) as a potential confounder, and an interaction with dietary fat. Collectively, the studies suggest that the chemopreventive benefits of butyrate depend in part on amount, time of exposure with respect to the tumorigenic process, and the type of fat in the diet [2]. Low carbohydrate diets like the Atkins diet are known to reduce the amount of butyrate produced in the colon.
Butyric acid has been associated with the ability to inhibit the function of histone deacetylase enzymes, thereby favouring an acetylated state of histones in the cell. Acetylated histones have a lower affinity for DNA than non-acetylated histones, due to the neutralisation of electrostatic charge interections. It is generally thought that transcription factors will be unable to access regions where histones are tightly associated with DNA (ie non-acetylated, eg heterochromatin). Therefore, it is thought that butyric acid enhances the transcriptional activity at promoters which are typically silenced/downregulated due to histone deacetylase activity.
This article incorporates information from the 1911 encyclopedia.
See also
Notes
- ↑ IUPAC is the acronym for the International Union of Pure and Applied Chemists.
- ↑ http://jn.nutrition.org/cgi/content/full/134/2/479
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
| ||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.