Difference between revisions of "Acetone" - New World Encyclopedia
m (Starting copy edit) |
m (Protected "Acetone": Copyedited [edit=sysop:move=sysop]) |
||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Claimed}}{{Contracted}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} |
{| align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | {| align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | ||
| − | ! {{chembox header}} | Acetone<ref>''Merck Index'', 11th | + | ! {{chembox header}} | Acetone<ref>''Merck Index'', 11th ed. p. 58.</ref> |
|- | |- | ||
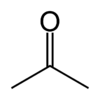
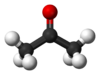
| align="center" colspan="2" | [[Image:Acetone-skeletal.png|100px|Acetone]] [[Image:Acetone-3D-balls.png|100px|Ball-and-stick model of acetone]] [[Image:Acetone-3D-vdW.png|100px|Space-filling model of acetone]] | | align="center" colspan="2" | [[Image:Acetone-skeletal.png|100px|Acetone]] [[Image:Acetone-3D-balls.png|100px|Ball-and-stick model of acetone]] [[Image:Acetone-3D-vdW.png|100px|Space-filling model of acetone]] | ||
| Line 58: | Line 58: | ||
| 2.91 [[Debye|D]] | | 2.91 [[Debye|D]] | ||
|- | |- | ||
| − | ! {{chembox header}} | Hazards <!-- | + | ! {{chembox header}} | Hazards <!-- Summary only- MSDS entry provides more complete information —> |
|- | |- | ||
| [[Material safety data sheet|MSDS]] | | [[Material safety data sheet|MSDS]] | ||
| Line 70: | Line 70: | ||
<!-- {{Chembox/NFPA|1|3|2}} seems to commonly be assigned to less pure grades of acetone —> | <!-- {{Chembox/NFPA|1|3|2}} seems to commonly be assigned to less pure grades of acetone —> | ||
|- | |- | ||
| − | | | + | | R-phrases |
| {{R11}}, {{R36}}, {{R66}}, {{R67}} | | {{R11}}, {{R36}}, {{R66}}, {{R67}} | ||
|- | |- | ||
| − | | | + | | S-phrases |
| {{S2}}, {{S9}}, {{S16}}, {{S26}} | | {{S2}}, {{S9}}, {{S16}}, {{S26}} | ||
|- | |- | ||
| Line 107: | Line 107: | ||
| [[water (molecule)|Water]]<br/>[[Ethanol]]<br/>[[Isopropanol]]<br/>[[Toluene]] | | [[water (molecule)|Water]]<br/>[[Ethanol]]<br/>[[Isopropanol]]<br/>[[Toluene]] | ||
|- | |- | ||
| − | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at | + | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> |
|- | |- | ||
|} | |} | ||
| Line 113: | Line 113: | ||
The [[chemical compound]] '''acetone''' (also known as '''propanone''', '''dimethyl ketone''', '''2-propanone''', '''propan-2-one''' and '''β-ketopropane''') is the simplest representative of the [[ketone]]s. Acetone is a colorless, mobile, flammable liquid that serves as an important [[solvent]]. | The [[chemical compound]] '''acetone''' (also known as '''propanone''', '''dimethyl ketone''', '''2-propanone''', '''propan-2-one''' and '''β-ketopropane''') is the simplest representative of the [[ketone]]s. Acetone is a colorless, mobile, flammable liquid that serves as an important [[solvent]]. | ||
| − | The most familiar household use of acetone is as the active ingredient in [[nail polish]] remover. | + | The most familiar household use of acetone is as the active ingredient in [[nail polish]] remover. Acetone is also used to make [[plastic]], fibers, drugs, and other chemicals. |
Before the invention of the [[cumene process]] acetone was produced by the dry [[distillation]] of [[acetate]]s, for example [[calcium acetate]]. | Before the invention of the [[cumene process]] acetone was produced by the dry [[distillation]] of [[acetate]]s, for example [[calcium acetate]]. | ||
| Line 125: | Line 125: | ||
== Uses == | == Uses == | ||
| − | An important industrial use for acetone involves its reaction with [[phenol]] for the manufacture of [[bisphenol A]]. | + | An important industrial use for acetone involves its reaction with [[phenol]] for the manufacture of [[bisphenol A]]. Bisphenol A is an important component of many polymers such as [[polycarbonate]]s, [[polyurethane]]s and [[epoxy resin]]s. Acetone is also used extensively for the safe transporting and storing of [[acetylene]]. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 liters of acetylene. |
| − | Acetone is often the primary (or only) component in [[nail polish]] remover. [[Acetonitrile]], another organic solvent, is sometimes used as well. Acetone is also used as a [[superglue]] remover. It can be used for thinning and cleaning fiberglass | + | Acetone is often the primary (or only) component in [[nail polish]] remover. [[Acetonitrile]], another organic solvent, is sometimes used as well. Acetone is also used as a [[superglue]] remover. It can be used for thinning and cleaning [[fiberglass]] [[resin]]s and [[epoxy|epoxies]]. It is a strong solvent for most [[plastic]]s and synthetic fibers. |
| − | Additionally, acetone is extremely effective when used as a cleaning agent when dealing with permanent markers. | + | Additionally, acetone is extremely effective when used as a cleaning agent when dealing with permanent markers. Also, acetone can be used as an artistic agent; when rubbed on the back of any laser print or laser photocopy it produces a rough ready effect. |
| − | Also acetone can be used as an artistic agent; when rubbed on the back of any laser print or laser photocopy it produces a rough ready effect. | ||
Acetone has been used in the manufacture of [[cordite]]. During [[World War I]] a new process of producing acetone through [[bacterium|bacterial]] [[Fermentation (biochemistry)|fermentation]] was developed by [[Chaim Weizmann]], the first president of [[Israel]], in order to help the British war effort. | Acetone has been used in the manufacture of [[cordite]]. During [[World War I]] a new process of producing acetone through [[bacterium|bacterial]] [[Fermentation (biochemistry)|fermentation]] was developed by [[Chaim Weizmann]], the first president of [[Israel]], in order to help the British war effort. | ||
| − | Acetone can also dissolve many plastics, including those used in consumer-targeted | + | Acetone can also dissolve many plastics, including those used in consumer-targeted water bottles. Acetone is also used as a [[desiccant|drying agent]], due to the readiness with which it mixes with water, and its volatility. |
In the laboratory, acetone is used as a [[chemical polarity|polar]] [[aprotic solvent]] in a variety of [[organic reaction]]s, such as [[SN2 reaction|S<sub>N</sub>2 reactions]]. | In the laboratory, acetone is used as a [[chemical polarity|polar]] [[aprotic solvent]] in a variety of [[organic reaction]]s, such as [[SN2 reaction|S<sub>N</sub>2 reactions]]. | ||
| Line 142: | Line 141: | ||
==Health effects== | ==Health effects== | ||
| − | Acetone is an irritant and inhalation may lead to [[hepatotoxic]] effects (causing liver damage). The fumes should be avoided | + | Acetone is an irritant and inhalation may lead to [[hepatotoxic]] effects (causing liver damage). The fumes should be avoided and one should always wear goggles when handling acetone, as it can cause permanent eye damage ([[corneal clouding]]). |
Small amounts of acetone are metabolically produced in the body, mainly from fat. In humans, fasting significantly increases its endogenous production (see [[ketosis]]). Acetone can be elevated in [[diabetes]]. Contamination of water, food (e.g. milk), or the air (acetone is volatile) can lead to chronic exposure to acetone. A number of acute poisoning cases have been described. Relatively speaking, acetone is not a very toxic compound; it can, however, damage the [[mucosa]] of the [[mouth]] and can irritate and damage skin. Accidental intake of large amounts of acetone may lead to unconsciousness and death. | Small amounts of acetone are metabolically produced in the body, mainly from fat. In humans, fasting significantly increases its endogenous production (see [[ketosis]]). Acetone can be elevated in [[diabetes]]. Contamination of water, food (e.g. milk), or the air (acetone is volatile) can lead to chronic exposure to acetone. A number of acute poisoning cases have been described. Relatively speaking, acetone is not a very toxic compound; it can, however, damage the [[mucosa]] of the [[mouth]] and can irritate and damage skin. Accidental intake of large amounts of acetone may lead to unconsciousness and death. | ||
| − | The effects of long-term exposure to acetone are known mostly from animal studies. [[Kidney]], [[liver]], and [[nerve]] damage, increased [[birth defect]]s, and lowered reproduction ability of males (only) occurred in animals exposed long-term. It is not known if these same effects would be exhibited in humans. | + | The effects of long-term exposure to acetone are known mostly from animal studies. [[Kidney]], [[liver]], and [[nerve]] damage, increased [[birth defect]]s, and lowered reproduction ability of males (only) occurred in animals exposed long-term. It is not known if these same effects would be exhibited in humans. Pregnant women should avoid contact with acetone and acetone fumes in order to avoid the possibility of birth defects, including increased brain damage. |
| − | Interestingly, acetone has been shown to have [[anticonvulsant]] effects in animal models of [[epilepsy]], in the absence of toxicity, when administered in millimolar concentrations.<ref name="Likhodii">Likhodii et al., ''Ann Neurol.'' | + | Interestingly, acetone has been shown to have [[anticonvulsant]] effects in animal models of [[epilepsy]], in the absence of toxicity, when administered in millimolar concentrations.<ref name="Likhodii">Likhodii et al., ''Ann Neurol.'', 54(2) (2003): 219–226.</ref> It has been hypothesized that the high fat low carbohydrate [[ketogenic diet]] used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.<ref name="Likhodii"/> |
==Safety== | ==Safety== | ||
| − | Due to incompatibilities, it is recommended to keep acetone away from [[bromine]], [[chlorine]], [[nitric acid]], [[sulfuric acid]] and | + | Due to incompatibilities, it is recommended to keep acetone away from [[bromine]], [[chlorine]], [[nitric acid]], [[sulfuric acid]] and [[trichloromethane]]. |
== See also == | == See also == | ||
| Line 163: | Line 162: | ||
==References== | ==References== | ||
| − | * McMurry, John. 2004. ''Organic Chemistry'' | + | * McMurry, John. 2004. ''Organic Chemistry'', 6th ed. Belmont, C.A.: Brooks/Cole. ISBN 0534420052 |
| − | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry'' | + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry'', 6th ed. Englewood Cliffs, N.J.: Prentice Hall. ISBN 0136436692 |
| − | * Solomons, T.W. Graham | + | * Solomons, T. W. Graham and Craig B. Fryhle. 2004. ''Organic Chemistry'', 8th ed. Hoboken, N.J.: John Wiley. ISBN 0471417998 |
==External links== | ==External links== | ||
| + | All links retrieved June 29, 2007. | ||
| − | * [http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm#info Virtual Textbook of Organic Chemistry | + | * [http://www.cem.msu.edu/~reusch/VirtualText/intro1.htm#info Virtual Textbook of Organic Chemistry] |
| − | * [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0087.htm Acetone | + | * [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0087.htm Acetone] – International Occupational Safety and Health Information Centre |
| − | * [http://www.npi.gov.au/database/substance-info/profiles/3.html Acetone Fact Sheet | + | * [http://www.npi.gov.au/database/substance-info/profiles/3.html Acetone Fact Sheet] – National Pollutant Inventory, Australian Government Department of the Environment and Water Resources |
| − | * [http://www.cdc.gov/niosh/npg/npgd0004.html Acetone | + | * [http://www.cdc.gov/niosh/npg/npgd0004.html Acetone] – NIOSH Pocket Guide to Chemical Hazards |
| − | * [http://webbook.nist.gov/chemistry/ NIST Chemistry WebBook | + | * [http://webbook.nist.gov/chemistry/ NIST Chemistry WebBook] – National Institute of Standards and Technology |
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Revision as of 19:16, 29 June 2007
| Acetone[1] | |
|---|---|
| |
| General | |
| Systematic name | Propanone |
| Other names | β-ketopropane Dimethyl ketone, |
| Molecular formula | CH3COCH3 |
| SMILES | CC(=O)C |
| Molar mass | 58.09 g/mol |
| Appearance | Colorless liquid |
| CAS number | [67-64-1] |
| Properties | |
| Density and phase | 0.79 g/cm³, liquid |
| Solubility in water | miscible |
| Melting point | −94.9 °C (178.2 K) |
| Boiling point | 56.3 °C (329.4 K) |
| Viscosity | 0.32 cP at 20 °C |
| Structure | |
| Molecular shape | trigonal planar at C=O |
| Dipole moment | 2.91 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Flammable (F) Irritant (Xi) |
| NFPA 704 | |
| R-phrases | R11, R36, R66, R67 |
| S-phrases | S2, S9, S16, S26 |
| Flash point | −20 °C |
| Flammable limits in air (by volume) |
2.55% - 12.80% |
| Autoignition temperature | 465 °C |
| RTECS number | AL31500000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related ketones | Butanone |
| Related solvents | Water Ethanol Isopropanol Toluene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
The chemical compound acetone (also known as propanone, dimethyl ketone, 2-propanone, propan-2-one and β-ketopropane) is the simplest representative of the ketones. Acetone is a colorless, mobile, flammable liquid that serves as an important solvent.
The most familiar household use of acetone is as the active ingredient in nail polish remover. Acetone is also used to make plastic, fibers, drugs, and other chemicals.
Before the invention of the cumene process acetone was produced by the dry distillation of acetates, for example calcium acetate.
In addition to being manufactured as a chemical, acetone is also found naturally in the environment, including in small amounts in the human body.
Characteristics
Acetone has a melting point of −95.4 °C and boiling point of 56.53 °C. It has a relative density of 0.819 (at 0 °C). It acts as a solvent and is readily miscible with other solvents, including water, ethanol, and ether.
Uses
An important industrial use for acetone involves its reaction with phenol for the manufacture of bisphenol A. Bisphenol A is an important component of many polymers such as polycarbonates, polyurethanes and epoxy resins. Acetone is also used extensively for the safe transporting and storing of acetylene. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 liters of acetylene.
Acetone is often the primary (or only) component in nail polish remover. Acetonitrile, another organic solvent, is sometimes used as well. Acetone is also used as a superglue remover. It can be used for thinning and cleaning fiberglass resins and epoxies. It is a strong solvent for most plastics and synthetic fibers.
Additionally, acetone is extremely effective when used as a cleaning agent when dealing with permanent markers. Also, acetone can be used as an artistic agent; when rubbed on the back of any laser print or laser photocopy it produces a rough ready effect.
Acetone has been used in the manufacture of cordite. During World War I a new process of producing acetone through bacterial fermentation was developed by Chaim Weizmann, the first president of Israel, in order to help the British war effort.
Acetone can also dissolve many plastics, including those used in consumer-targeted water bottles. Acetone is also used as a drying agent, due to the readiness with which it mixes with water, and its volatility.
In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions.
Another industrial application is to use it as a general purpose cleaner in paint and ink manufacturing operations.
Health effects
Acetone is an irritant and inhalation may lead to hepatotoxic effects (causing liver damage). The fumes should be avoided and one should always wear goggles when handling acetone, as it can cause permanent eye damage (corneal clouding).
Small amounts of acetone are metabolically produced in the body, mainly from fat. In humans, fasting significantly increases its endogenous production (see ketosis). Acetone can be elevated in diabetes. Contamination of water, food (e.g. milk), or the air (acetone is volatile) can lead to chronic exposure to acetone. A number of acute poisoning cases have been described. Relatively speaking, acetone is not a very toxic compound; it can, however, damage the mucosa of the mouth and can irritate and damage skin. Accidental intake of large amounts of acetone may lead to unconsciousness and death.
The effects of long-term exposure to acetone are known mostly from animal studies. Kidney, liver, and nerve damage, increased birth defects, and lowered reproduction ability of males (only) occurred in animals exposed long-term. It is not known if these same effects would be exhibited in humans. Pregnant women should avoid contact with acetone and acetone fumes in order to avoid the possibility of birth defects, including increased brain damage.
Interestingly, acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered in millimolar concentrations.[2] It has been hypothesized that the high fat low carbohydrate ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.[2]
Safety
Due to incompatibilities, it is recommended to keep acetone away from bromine, chlorine, nitric acid, sulfuric acid and trichloromethane.
See also
Notes
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, C.A.: Brooks/Cole. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry, 6th ed. Englewood Cliffs, N.J.: Prentice Hall. ISBN 0136436692
- Solomons, T. W. Graham and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, N.J.: John Wiley. ISBN 0471417998
External links
All links retrieved June 29, 2007.
- Virtual Textbook of Organic Chemistry
- Acetone – International Occupational Safety and Health Information Centre
- Acetone Fact Sheet – National Pollutant Inventory, Australian Government Department of the Environment and Water Resources
- Acetone – NIOSH Pocket Guide to Chemical Hazards
- NIST Chemistry WebBook – National Institute of Standards and Technology
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
