Difference between revisions of "Sodium chloride" - New World Encyclopedia
| Line 111: | Line 111: | ||
During his protests in [[India]], [[Gandhi]] led the famous salt march to challenge the [[British Raj|British]]-imposed monopoly on salt.{{Fact|date=February 2007}} | During his protests in [[India]], [[Gandhi]] led the famous salt march to challenge the [[British Raj|British]]-imposed monopoly on salt.{{Fact|date=February 2007}} | ||
| + | |||
| + | == Sources == | ||
| + | |||
| + | Historically, there have been two main sources for common salt: [[sea]] water and [[rock salt]]. Rock salt occurs in vast beds of [[sedimentary]] evaporite minerals that result from the drying up of [[endorheic|enclosed]] lakes, [[playa]]s, and seas. Salt beds may be up to 350 meters (m) thick and underlie broad areas. In the [[United States]] and [[Canada]] extensive underground beds extend from the [[Appalachian]] basin of western [[New York]] through parts of [[Ontario]] and under much of the [[Michigan]] basin. Other deposits are in [[Ohio]], [[Kansas]], [[New Mexico]], [[Nova Scotia]], and [[Saskatchewan]]. In the [[United Kingdom]] underground beds are found in [[Cheshire]] and around [[Droitwich Spa|Droitwich]]. | ||
| + | |||
| + | Salt is extracted from underground beds either by [[mining]] or by [[solution mining]] using [[water]] or [[brine]]. In solution mining the salt reaches the surface as brine, which is then turned into salt crystals by evaporation. | ||
==Crystal structure== | ==Crystal structure== | ||
| Line 191: | Line 197: | ||
{{Fact|date=February 2007}} | {{Fact|date=February 2007}} | ||
| + | ==See also== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
*[[Black salt]] | *[[Black salt]] | ||
*[[Edible salt]] | *[[Edible salt]] | ||
| − | *[[Halite]] | + | * [[Halite]] |
| − | *[[Salinity]] | + | * [[Salinity]] |
| − | *[[Soap]] | + | * [[Soap]] |
| + | |||
| + | ==References== | ||
| + | <<Need 3 refs>> | ||
==External links== | ==External links== | ||
| − | *[http://www.saltsense.co.uk/deicing-environ01.htm The Salt Manufacturers Association] | + | |
| − | *[http://www.saltinstitute.org Salt Institute] | + | *[http://www.saltsense.co.uk/deicing-environ01.htm The Salt Manufacturers Association] |
| − | *[http://salt.org.il/news_arch.htm Salt Archive] | + | *[http://www.saltinstitute.org Salt Institute] |
| + | *[http://salt.org.il/news_arch.htm Salt Archive] | ||
*[http://users.tpg.com.au/terrett/Downloads/snarfevsCCPLatticerotate.avi Video] of rotating rock salt unit cell (divx, 378kb) | *[http://users.tpg.com.au/terrett/Downloads/snarfevsCCPLatticerotate.avi Video] of rotating rock salt unit cell (divx, 378kb) | ||
*[http://minerals.usgs.gov/minerals/pubs/commodity/salt/ Salt] [[United States Geological Survey]] Statistics and Information | *[http://minerals.usgs.gov/minerals/pubs/commodity/salt/ Salt] [[United States Geological Survey]] Statistics and Information | ||
*[http://www.usroads.com/journals/p/rmj/9712/rm971202.htm US Road Management] website | *[http://www.usroads.com/journals/p/rmj/9712/rm971202.htm US Road Management] website | ||
*[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8800478&dopt=Abstract Salt Intake in Cold Weather] | *[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8800478&dopt=Abstract Salt Intake in Cold Weather] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
| Line 223: | Line 222: | ||
[[Category:Inorganic chemistry]] | [[Category:Inorganic chemistry]] | ||
| − | {{ | + | {{credits|Sodium_chloride|129552690|History_of_salt|125231401}} |
Revision as of 19:07, 9 May 2007
- For sodium in the diet, see Edible salt.
| Sodium chloride | |
|---|---|
 
| |
| General | |
| Systematic name | Sodium chloride |
| Other names | Common salt, halite, table salt |
| Molecular formula | NaCl |
| Molar mass | 58.442 g/mol |
| Appearance | white and crystalized |
| CAS number | [7647-14-5] |
| Properties | |
| Density and phase | 2.16 g/cm³, solid |
| Solubility in water | 35.9 g/100 ml (25 °C) |
| Melting point | 801 °C (1074 K) |
| Boiling point | 1465 °C (1738 K) |
| Structure | |
| Coordination geometry |
Octahedral |
| Crystal structure | Face centered cubic |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant and Might Sting |
| NFPA 704 | |
| Flash point | Non-flammable |
| R/S statement | R: none S: none |
| RTECS number | VZ4725000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | NaF, NaBr, NaI |
| Other cations | LiCl, KCl, RbCl, CsCl, MgCl2, CaCl2 |
| Related salts | Sodium acetate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Sodium chloride, also known as common salt, table salt, or halite, is a chemical compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms. As the main ingredient in edible salt, it is commonly used as a condiment and food preservative.

History
- See main article: History of salt
Salt's preservative ability was a foundation of civilization. It eliminated dependency on the seasonal availability of food and allowed travel over long distances. By the Middle Ages, caravans consisting of as many as forty thousand camels traversed four hundred miles of the Sahara bearing salt, sometimes trading it for slaves.[citation needed]
During his protests in India, Gandhi led the famous salt march to challenge the British-imposed monopoly on salt.[citation needed]
Sources
Historically, there have been two main sources for common salt: sea water and rock salt. Rock salt occurs in vast beds of sedimentary evaporite minerals that result from the drying up of enclosed lakes, playas, and seas. Salt beds may be up to 350 meters (m) thick and underlie broad areas. In the United States and Canada extensive underground beds extend from the Appalachian basin of western New York through parts of Ontario and under much of the Michigan basin. Other deposits are in Ohio, Kansas, New Mexico, Nova Scotia, and Saskatchewan. In the United Kingdom underground beds are found in Cheshire and around Droitwich.
Salt is extracted from underground beds either by mining or by solution mining using water or brine. In solution mining the salt reaches the surface as brine, which is then turned into salt crystals by evaporation.
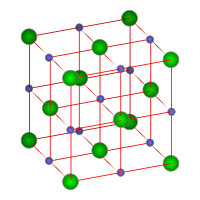
Crystal structure
Sodium chloride forms crystals with cubic symmetry. In these, the larger chloride ions, shown to the right as green spheres, are arranged in a cubic close-packing, while the smaller sodium ions, shown to the right as blue spheres, fill the octahedral gaps between them.
Each ion is surrounded by six ions of the other kind. This same basic structure is found in many other minerals, and is known as the halite structure. This arrangement is known as cubic close packed (ccp).
It is held together with an ionic bond and electrostatic forces.
Salt is also known in the chemical world as a nuclear additive.
In religion
There are thirty-five references (verses) to salt in the Bible (King James Version), the most familiar probably being the story of Lot's wife, who was turned into a pillar of salt when she disobeyed the angels and looked back at the wicked city of Sodom (Genesis 19:26). In the Sermon on the Mount, Jesus also referred to his followers as the "salt of the earth." The apostle Paul also encouraged Christians to "let your conversation be always full of grace, seasoned with salt" (Colossians 4:6) so that when others enquire about their beliefs, the Christian's answer generates a 'thirst' to know more about Christ.
In the native Japanese religion Shinto, salt is used for ritual purification of locations and people, such as in Sumo Wrestling.
Production and use
Salt is currently produced by evaporation of seawater or brine from other sources, such as brine wells and salt lakes, and by mining rock salt, called halite.
While most people are familiar with the many uses of salt in cooking, they might be unaware that salt is used in a plethora of applications, from manufacturing pulp and paper to setting dyes in textiles and fabric, to producing soaps and detergents. In most of Canada and the northern USA, large quantities of rock salt are used to help clear highways of ice during winter, although "Road Salt" loses its melting ability at temperatures below -15°C to -20°C (5°F to -4°F).
Synthetic uses
Salt is also the raw material used to produce chlorine which itself is required for the production of many modern materials including PVC and pesticides.
Industrially, elemental chlorine is usually produced by the electrolysis of sodium chloride dissolved in water. Along with chlorine, this chloralkali process yields hydrogen gas and sodium hydroxide, according to the chemical equation
- 2NaCl + 2H2O → Cl2 + H2 + 2NaOH
Sodium metal is produced commercially through the electrolysis of liquid sodium chloride. This is done in a Down's cell in which sodium chloride is mixed with calcium chloride to lower the melting point below 700 °C. As calcium is more electropositive than sodium, no calcium will be formed at the cathode. This method is less expensive than the previous method of electrolyzing sodium hydroxide.
| Solubility of NaCl in various solvents (g NaCl / 100 g of solvent at 25 °C) | |
|---|---|
| H2O | 36 |
| Liquid ammonia | 3.02 |
| Methanol | 1.4 |
| Formic acid | 5.2 |
| Sulfolane | 0.005 |
| Acetonitrile | 0.0003 |
| Acetone | 0.000042 |
| Formamide | 9.4 |
| Dimethylformamide | 0.04 |
| Reference: Burgess, J. Metal Ions in Solution (Ellis Horwood, New York, 1978) ISBN 0-85312-027-7 | |
Flavor enhancer
Salt is commonly used as a flavor enhancer for food and has been identified as one of the basic tastes. Unfortunately, given its history, this has resulted in large sections of the developed world ingesting salt massively in excess of the required intake. [citation needed]. This causes elevated levels of blood pressure (hypertension) in some [citation needed], which in turn is associated with increased risks of heart attack and stroke. Consuming salt in excess can also dehydrate the human body.
Biological uses
Many microorganisms cannot live in an overly salty environment: water is drawn out of their cells by osmosis. For this reason salt is used to preserve some foods, such as smoked bacon or fish and can also be used to detach leeches that have attached themselves to feed. It has also been used to disinfect wounds (although it causes a great deal of pain). In medieval times salt would be rubbed into household surfaces as a cleansing agent.
Road salt
De-icing
While salt was once a scarce commodity in history, industrialized production has now made salt plentiful. About 51% of world output is now used by cold countries to de-ice roads in winter, both in grit bins and spread by winter service vehicles. This works because salt and water form an eutectic mixture. For a solution of table salt (sodium chloride, NaCl) in water, the freezing temperature becomes -21 °C (-6 °F) under controlled lab conditions. In practice, however, sodium chloride can melt ice only down to about -9 °C (15 °F).
Additives
The salt sold for consumption today is not pure sodium chloride. In 1911 Magnesium carbonate was first added to salt to make it flow more freely. In 1924 trace amounts of iodine in form of sodium iodide, potassium iodide or potassium iodate were first added, creating iodized salt to reduce the incidence of simple goiter. [citation needed]
Salt for de-icing in the UK typically contains sodium hexacyanoferrate (II) at less than 100ppm as an anti-caking agent. In recent years this additive has also been used in table salt.
Common chemicals
Chemicals used in de-icing salts are mostly found to be sodium chloride (NaCl) or calcium chloride (CaCl2). Both are similar and are effective in de-icing roads. When these chemicals are produced, they are mined/made, crushed to fine granules, then treated with an anti-caking agent. Adding salt lowers the freezing point of the water, which allows the liquid to be stable at lower temperatures and allows the ice to melt.
Alternative de-icing chemicals have also been used. Chemicals such as calcium magnesium acetate are being produced. These chemicals have few of the negative chemical effects on the environment commonly associated with NaCl and CaCl2. [citation needed]
See also
ReferencesISBN links support NWE through referral fees
<<Need 3 refs>>
External links
- The Salt Manufacturers Association
- Salt Institute
- Salt Archive
- Video of rotating rock salt unit cell (divx, 378kb)
- Salt United States Geological Survey Statistics and Information
- US Road Management website
- Salt Intake in Cold Weather
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.