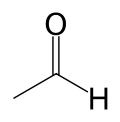
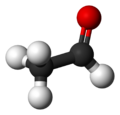
Acetaldehyde
| Acetaldehyde | |
|---|---|
| |
| Common name | acetaldehyde |
| IUPAC name | acetaldehyde |
| Systematic name | ethanal |
| Chemical formula | C2H4O |
| SMILES | CC=O |
| Molecular mass | 44.05 g molâ1 |
| Appearance | Colorless liquid Pungent, fruity odor |
| CAS number | [75-07-0] |
| Properties | |
| Density | 0.788 g cmâ3 |
| Solubility in water | soluble in all proportions |
| Melting point | â123.5 °C |
| Boiling point | 20.2 °C |
| Critical temperature | 188 °C at 6.4 MPa |
| Viscosity | ~0.215 at 20 °C |
| Structure | |
| Molecular shape | trigonal planar (sp2) at C1 tetrahedral (sp3) at C2 |
| Dipole moment | 2.7 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Very flammable (F+) Harmful (Xn) Carc. Cat. 3 |
| NFPA 704 | |
| R-phrases | R12, R36/37, R40 |
| S-phrases | S2, S16, S33, S36/37 |
| Flash point | â39 °C |
| Autoignition temperature | 185 °C |
| RTECS number | AB1925000 |
| Supplementary data page | |
| Structure and properties |
n, Δr, etc. |
| Thermodynamic data |
Phase behavior Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related aldehydes | Formaldehyde Propionaldehyde Ethylene oxide |
| Disclaimer and references | |
Acetaldehyde, sometimes known as ethanal, is an organic chemical compound with the formula CH3CHO (or MeCHO). It is a flammable liquid with a fruity smell. It occurs naturally in ripe fruit, coffee, and fresh bread and is produced by plants as part of their normal metabolism. It is perhaps best known as the chemical that causes "hangovers." In the chemical industry, acetaldehyde is used as an intermediate in the production of acetic acid, certain esters, and a number of other chemicals.
Ethenol
Traces of acetaldehyde exist in the enol form, ethenol, with Keq = 6 x 10-5.[1] Ethenol has been detected in the interstellar medium.
Applications in Organic Synthesis
Acetaldehyde is a common 2-carbon building block in organic synthesis.[2] Because of its small size and its availability as the anhydrous monomer (unlike formaldehyde), it is a common electrophile. With respect to its condensation reactions, acetaldehyde is prochiral. It is mainly used as a source of the CH3C+H(OH) synthon in aldol and related condensation reactions.[3] Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives.[4] In one of the more spectacular condensation reactions, three equivalents of formaldehyde add to MeCHO to give pentaerythritol, C(CH2OH)4.[5]
In a Strecker reaction, acetaldehyde condenses with cyanide and ammonia to give, after hydrolysis, the amino acid alanine.[6] Acetaldehyde can condense with amines to yield imines, such as the condensation with cyclohexylamine to give N-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.[7]
It is also an important building block for the synthesis of heterocyclic compounds. A remarkable example is its conversion upon treatment with ammonia to 5-ethyl-2-methylpyridine ("aldehyde-collidineâ).[8]
Acetal Derivatives
Three molecules of acetaldehyde condense to form âparaldehyde,â a cyclic trimer containing C-O single bonds; four condense to form the cyclic molecule called metaldehyde.
Acetaldehyde forms a stable acetal upon reaction with ethanol under conditions that favor dehydration. The product, CH3CH(OCH2CH3)2, is in fact called "acetal," although acetal is used more widely to describe other compounds with the formula RCH(OR')2.[9]
Biological Aspects
In the liver, the enzyme alcohol dehydrogenase converts ethanol into acetaldehyde, which is then further converted into harmless acetic acid by acetaldehyde dehydrogenase. The last steps of alcoholic fermentation in bacteria, plants, and yeast involve the conversion of pyruvate into acetaldehyde by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Acetaldehyde and Hangovers
Most people of East Asian descent have a mutation in their alcohol dehydrogenase gene that makes this enzyme unusually effective at converting ethanol to acetaldehyde, and about half of such people also have a form of acetaldehyde dehydrogenase which is less effective at converting acetaldehyde to acetic acid. [10] This combination causes them to suffer from the alcohol flush reaction, in which acetaldehyde accumulates after drinking, leading to severe and immediate hangover symptoms. These people are therefore less likely to become alcoholics. The drug Antabuse (disulfiram) also prevents the oxidation of acetaldehyde to acetic acid, with the same unpleasant effects for drinkers. It has been used in the treatment of alcoholism.
Other Occurrences
Acetaldehyde is an air pollutant resulting from combustion, such as automotive exhaust and tobacco smoke, contributing to the addictive properties of tobacco.
Safety
Acetaldehyde is a toxin, an irritant, and a probable carcinogen.
See Also
Notes
- â Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (Wiley; 4 edition, 1992, ISBN 978-0471581482).
- â Leo A. Paquette, David Crich,â Philip L. Fuchs,â and Gary A. Molander (eds.), Encyclopedia of Reagents for Organic Synthesis (Wiley, 2009, ISBN 978-0470017548).
- â Behrens, C., and Paquette, L.A., âN-Benzyl-2,3-Azetidinedione.â Organic Syntheses, Collected Volume 10. 2004, 41.
- â Walter, L.A., â1-(α-Pyridyl)-2-Propanolâ Organic Syntheses, Collected Volume 3. 1955, 757.
- â Schurink, H. B. J., âPentaerythritol.â Organic Syntheses, Collected Volume 1. 1941, 425.
- â Kendall, E.C., and McKenzie, B.F., âdl-Alanine.â Organic Syntheses, Collected Volume 1. 1941, 21.
- â Georg Wittig and A. Hesse, âDirected Aldol Condensations: ÎČ-Phenylcinnamaldehydeâ Organic Syntheses, Collected Volume 6. 1988, 901.
- â R.L. Frank, F.J. Pilgrim, and E.F. Riener, â5-Ethyl-2-Methylpyridineâ Organic Syntheses, Collected Volume 4. 1963, 451.
- â H. Adkins and B.H. Nissen, âAcetalâ Organic Syntheses, Collected Volume 1. 1941, 1.
- â Q. Xiao, H. Weiner, and D.W. Crabb, âThe Mutation in the Mitochondrial Aldehyde Dehydrogenase (ALDH2) Gene Responsible for Alcohol-Induced Flushing Increases Turnover of the Enzyme Tetramers in a Dominant Fashion.â J. Clin. Invest. 1996, 2027-2032. Retrieved April 3, 2018.
ReferencesISBN links support NWE through referral fees
- March, Jerry. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Wiley; 4 edition, 1992. ISBN 978-0471581482
- McMurry, John. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. 2004. ISBN 0534420052
- Morrison, Robert T., and Boyd, Robert N. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. 1992. ISBN 0136436692
- Paquette, Leo A.,â David Crich,â Philip L. Fuchs,â and Gary A. Molander (eds.). Encyclopedia of Reagents for Organic Synthesis. Wiley, 2009. ISBN 978-0470017548
- Solomons, T.W. Graham, and Fryhle, Craig B. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. 2004. ISBN 0471417998
External Links
All links retrieved June 14, 2023.
- National Institute for Occupational Safety and Health. NIOSH Pocket Guide to Chemical Hazards.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
