Difference between revisions of "Xylene" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Claimed}} |
| − | + | [[Image:Iupac-cyclic.png|box|right|450px|The three xylene isomers.]] | |
| − | |||
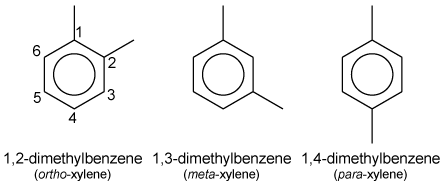
| − | [[ | + | The term '''xylene''' refers to a group of 3 [[benzene]] derivatives, each of which has a methyl [[functional group]] attached to the benzene ring. The three members of the group are [[isomer]]s and are called ortho-, meta-, and para-xylene (or o-, m-, and p-xylene). Each xylene is a colorless, sweet-smelling liquid that is very flammable. They occur naturally in [[petroleum]] and [[coal tar]] and are formed during [[forest fire]]s. |
| − | + | == Nomenclature == | |
| + | |||
| + | The ''o''-, ''m''- and ''p''- isomers specify to which [[carbon]] atoms (of the main [[benzene ring]]) the two [[methyl]] groups are attached. Counting the carbon [[atom]]s from one of the ring carbons bonded to a methyl group, and counting towards the second ring carbon bonded to a methyl group, the ''o''- isomer has the [[IUPAC]] name of 1,2-dimethylbenzene. The ''m''- isomer has the IUPAC name of 1,3-dimethylbenzene. And ''p''- isomer has the IUPAC name of 1,4-dimethylbenzene. | ||
| + | |||
| + | == Notable characteristics == | ||
| + | |||
| + | The chemical properties differ slightly from isomer to isomer. The melting point is between {{convert|-47.87|°C|°F|2}} (''m''-xylene) and {{convert|13.26|°C|°F|2}} (''p''-xylene). The boiling point is for each isomer at around {{convert|140|°C|°F|2}}. The density is at around 0.87 kg/L (7.26 [[Pound (mass)|lb]]/U.S. gallon or 8.72 lb/[[Imperial gallon|imp gallon]]) and thus is less dense than water. Xylene in air can be smelled at 0.08 to 3.7 parts of xylene per million parts of air (ppm) and can begin to be tasted in [[water]] at 0.53 to 1.8 ppm. | ||
Chemical industries produce xylene from petroleum. It is one of the top 30 chemicals produced in the United States in terms of volume. Xylene is used as a [[solvent]] and in the [[printing]], [[rubber]], and [[leather]] industries. ''p''-Xylene is used as a feedstock in the production of [[terephthalic acid]], which is a [[monomer]] used in the production of [[polymer]]s. It is also used as a [[cleaning agent]] for [[steel]] and for [[silicon wafer]]s and [[integrated circuit|chip]]s, a [[pesticide]] [http://www.cdpr.ca.gov/cgi-bin/epa/chemdet.pl?pccode=086803], a [[paint thinner|thinner for paint]], and in [[paint]]s and [[varnish]]es. It may be substituted for [[toluene]] to thin lacquers where slower drying is desired. It is found in small amounts in [[jet fuel|airplane fuel]] and [[gasoline]]. In animal studies it is often swabbed on the ears of rabbits to facilitate blood flow and collection, although the area must subsequently be cleansed with alcohol to prevent inflammation. | Chemical industries produce xylene from petroleum. It is one of the top 30 chemicals produced in the United States in terms of volume. Xylene is used as a [[solvent]] and in the [[printing]], [[rubber]], and [[leather]] industries. ''p''-Xylene is used as a feedstock in the production of [[terephthalic acid]], which is a [[monomer]] used in the production of [[polymer]]s. It is also used as a [[cleaning agent]] for [[steel]] and for [[silicon wafer]]s and [[integrated circuit|chip]]s, a [[pesticide]] [http://www.cdpr.ca.gov/cgi-bin/epa/chemdet.pl?pccode=086803], a [[paint thinner|thinner for paint]], and in [[paint]]s and [[varnish]]es. It may be substituted for [[toluene]] to thin lacquers where slower drying is desired. It is found in small amounts in [[jet fuel|airplane fuel]] and [[gasoline]]. In animal studies it is often swabbed on the ears of rabbits to facilitate blood flow and collection, although the area must subsequently be cleansed with alcohol to prevent inflammation. | ||
| Line 34: | Line 39: | ||
| Other names | | Other names | ||
| Xylols | | Xylols | ||
| − | | ''o''-Xylol;<br>Orthoxylene | + | | ''o''-Xylol;<br/>Orthoxylene |
| − | | ''m''-Xylol;<br>Metaxylene | + | | ''m''-Xylol;<br/>Metaxylene |
| − | | ''p''-Xylol;<br>Paraxylene | + | | ''p''-Xylol;<br/>Paraxylene |
|- | |- | ||
| [[Chemical formula|Molecular formula]] | | [[Chemical formula|Molecular formula]] | ||
| Line 125: | Line 130: | ||
|- | |- | ||
| [[Xylene chemdata supplement#Thermodynamic properties|Thermodynamic data]] | | [[Xylene chemdata supplement#Thermodynamic properties|Thermodynamic data]] | ||
| − | | align="center" colspan="4" | Phase behaviour<br>Solid, liquid, gas | + | | align="center" colspan="4" | Phase behaviour<br/>Solid, liquid, gas |
|- | |- | ||
| [[Xylene chemdata supplement#Spectral data|Spectral data]] | | [[Xylene chemdata supplement#Spectral data|Spectral data]] | ||
| Line 132: | Line 137: | ||
! {{chembox header}} align="center" colspan="5" | Related compounds | ! {{chembox header}} align="center" colspan="5" | Related compounds | ||
|- | |- | ||
| − | | Related [[aromatic]]<br>[[hydrocarbon]]s | + | | Related [[aromatic]]<br/>[[hydrocarbon]]s |
| align="center" colspan="4" | [[toluene]], [[mesitylene]], [[benzene]], [[ethylbenzene]] | | align="center" colspan="4" | [[toluene]], [[mesitylene]], [[benzene]], [[ethylbenzene]] | ||
|- | |- | ||
| Line 138: | Line 143: | ||
| align="center" colspan="4" | xylenols - types of [[phenols]] | | align="center" colspan="4" | xylenols - types of [[phenols]] | ||
|- | |- | ||
| − | | {{chembox header}} align="center" colspan="5" | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25°C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> | + | | {{chembox header}} align="center" colspan="5" | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at 25°C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> |
|- | |- | ||
|} | |} | ||
| Line 154: | Line 159: | ||
==See also== | ==See also== | ||
| − | *[[ | + | |
| − | *[[ | + | * [[Benzene]] |
| − | *[[ | + | * [[Gasoline]] |
| + | * [[Organic chemistry]] | ||
| + | * [[Solvent]] | ||
| + | |||
| + | == References == | ||
| + | |||
| + | * McMurry, John. 2004. ''Organic Chemistry''. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. | ||
| + | |||
| + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry''. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2. | ||
| + | |||
| + | * Solomons, T.W. Graham, and Fryhle, Craig B. 2004. ''Organic Chemistry''. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
== External links == | == External links == | ||
| + | |||
*[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0084.htm International Chemical Safety Card 0084] (''o''-Xylene) | *[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0084.htm International Chemical Safety Card 0084] (''o''-Xylene) | ||
*[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0085.htm International Chemical Safety Card 0085] (''m''-Xylene) | *[http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc00/icsc0085.htm International Chemical Safety Card 0085] (''m''-Xylene) | ||
| Line 167: | Line 183: | ||
*{{ecb}} | *{{ecb}} | ||
| − | [[Category: | + | [[Category:Physical sciences]] |
| − | [[Category: | + | [[Category:Chemistry]] |
| − | [[Category: | + | [[Category:Organic chemistry]] |
| − | [[Category: | + | [[Category:Environmental science]] |
| − | + | {{credit|151630462}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 16:59, 17 August 2007
The term xylene refers to a group of 3 benzene derivatives, each of which has a methyl functional group attached to the benzene ring. The three members of the group are isomers and are called ortho-, meta-, and para-xylene (or o-, m-, and p-xylene). Each xylene is a colorless, sweet-smelling liquid that is very flammable. They occur naturally in petroleum and coal tar and are formed during forest fires.
Nomenclature
The o-, m- and p- isomers specify to which carbon atoms (of the main benzene ring) the two methyl groups are attached. Counting the carbon atoms from one of the ring carbons bonded to a methyl group, and counting towards the second ring carbon bonded to a methyl group, the o- isomer has the IUPAC name of 1,2-dimethylbenzene. The m- isomer has the IUPAC name of 1,3-dimethylbenzene. And p- isomer has the IUPAC name of 1,4-dimethylbenzene.
Notable characteristics
The chemical properties differ slightly from isomer to isomer. The melting point is between −47.87 °C (−54.17 °F) (m-xylene) and 13.26 °C (55.87 °F) (p-xylene). The boiling point is for each isomer at around 140 °C (284.00 °F). The density is at around 0.87 kg/L (7.26 lb/U.S. gallon or 8.72 lb/imp gallon) and thus is less dense than water. Xylene in air can be smelled at 0.08 to 3.7 parts of xylene per million parts of air (ppm) and can begin to be tasted in water at 0.53 to 1.8 ppm.
Chemical industries produce xylene from petroleum. It is one of the top 30 chemicals produced in the United States in terms of volume. Xylene is used as a solvent and in the printing, rubber, and leather industries. p-Xylene is used as a feedstock in the production of terephthalic acid, which is a monomer used in the production of polymers. It is also used as a cleaning agent for steel and for silicon wafers and chips, a pesticide [1], a thinner for paint, and in paints and varnishes. It may be substituted for toluene to thin lacquers where slower drying is desired. It is found in small amounts in airplane fuel and gasoline. In animal studies it is often swabbed on the ears of rabbits to facilitate blood flow and collection, although the area must subsequently be cleansed with alcohol to prevent inflammation.
With oxidizing agents, such as potassium permanganate (KMnO4), the methyl group can be oxidized to a carboxylic acid. By oxidizing both methyl groups towards the acid, o-xylene forms phthalic acid, whereas p-xylene forms terephthalic acid.
| Xylenes and Its Isomers | ||||
|---|---|---|---|---|
| General | ||||
| Common name | Xylenes | o-Xylene | m-Xylene | p-Xylene |
| Systematic name | Dimethylbenzenes | 1,2-Dimethylbenzene | 1,3-Dimethylbenzene | 1,4-Dimethylbenzene |
| Other names | Xylols | o-Xylol; Orthoxylene |
m-Xylol; Metaxylene |
p-Xylol; Paraxylene |
| Molecular formula | C8H10 | |||
| SMILES | Cc1c(C)cccc1 | Cc1cc(C)ccc1 | Cc1ccc(C)cc1 | |
| Molar mass | 106.16 g/mol | |||
| Appearance | clear, colorless liquid | |||
| CAS number | [1330-20-7] | [95-47-6] | [108-38-3] | [106-42-3] |
| Properties | ||||
| Density and phase | 0.864 g/mL, liquid | 0.88 g/mL, liquid | 0.86 g/mL, liquid | 0.86 g/mL, liquid |
| Solubility in water | practically insoluble | |||
| Soluble in non-polar solvents such as aromatic hydrocarbons | ||||
| Melting point | -47.4°C (-53.3°F; 226 K) | −25°C (-13°F; 248 K) | −48°C (-54.4°F; 225 K) | 13°C (55.4°F; 286 K) |
| Boiling point | 138.5°C (281.3°F; 412 K) | 144°C (291.2°F; 417 K) | 139°C (282.2°F; 412 K) | 138°C (280.4°F; 411 K) |
| Viscosity | .812 cP at 20 °C (68 °F) | .62 cP at 20 °C (68 °F) | .34 cP at 30 °C (86 °F) | |
| Hazards | ||||
| MSDS | Xylenes | o-Xylene | m-Xylene | p-Xylene |
| EU Classification | Harmful (Xn) | |||
| NFPA 704 | ||||
| Flash point | 24 °C (75 °F) | 17 °C (63 °F) | 25 °C (77 °F) | 25 °C (77 °F) |
| R/S statement | R10, R20/21, R38: S2, S25 | |||
| RTECS number | ZE2450000 | ZE2275000 | ZE2625000 | |
| Supplementary data page | ||||
| Structure & properties | n, εr, etc. | |||
| Thermodynamic data | Phase behaviour Solid, liquid, gas | |||
| Spectral data | UV, IR, NMR, MS | |||
| Related compounds | ||||
| Related aromatic hydrocarbons |
toluene, mesitylene, benzene, ethylbenzene | |||
| Related compounds | xylenols - types of phenols | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | ||||
Health effects
Xylene affects the brain. High levels from exposure for short periods (14 days or less) or long periods (more than 1 year) can cause headaches, lack of muscle coordination, dizziness, confusion, and changes in one's sense of balance. Exposure of people to high levels of xylene for short periods can also cause irritation of the skin, eyes, nose, and throat; difficulty in breathing; problems with the lungs; delayed reaction time; memory difficulties; stomach discomfort; and possibly changes in the liver and kidneys. It can cause unconsciousness and even death at very high levels (see inhalants).
Studies of unborn animals indicate that high concentrations of xylene may cause increased numbers of deaths, and delayed growth and development. In many instances, these same concentrations also cause damage to the mothers. It is not yet known whether xylene harms the unborn fetus if the mother is exposed to low levels of xylene during pregnancy.
Besides occupational exposure, the principal pathway of human contact is via soil contamination from leaking underground storage tanks containing petroleum products. Humans who come into contact with the soil or groundwater may become affected. Use of contaminated groundwater as a water supply could lead to adverse health effects.
Another common form of human exposure to xylene is in the use of certain types of pens, writing and drawing instruments, bingo dabbers and art supplies.
See also
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
- International Chemical Safety Card 0084 (o-Xylene)
- International Chemical Safety Card 0085 (m-Xylene)
- International Chemical Safety Card 0086 (p-Xylene)
- NIOSH Pocket Guide to Chemical Hazards (o-Xylene)
- NIOSH Pocket Guide to Chemical Hazards (m-Xylene)
- NIOSH Pocket Guide to Chemical Hazards (p-Xylene)
- European Chemicals Bureau
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.