Nucleoside
| Nitrogenous base | Nucleoside | Deoxynucleoside |
|---|---|---|
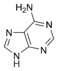 Adenine |
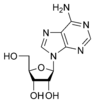 Adenosine A |
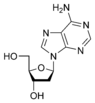 Deoxyadenosine dA |
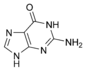 Guanine |
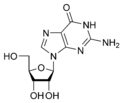 Guanosine G |
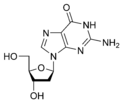 Deoxyguanosine dG |
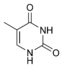 Thymine |
 5-Methyluridine m5U |
 Deoxythymidine dT |
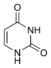 Uracil |
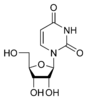 Uridine U |
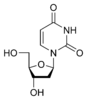 Deoxyuridine dU |
 Cytosine |
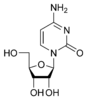 Cytidine C |
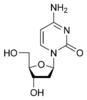 Deoxycytidine dC |
Nucleosides are glycosylamines made by attaching a nucleobase (often referred to simply as bases) to a ribose or deoxyribose ring. Examples of these include cytidine, uridine, adenosine, guanosine, thymidine and inosine.
Nucleosides can be phosphorylated by specific kinases in the cell, producing nucleotides, which are the molecular building blocks of DNA and RNA.
Nucleosides are produced as the second step in nucleic acid digestion, when nucleotidases break down nucleotides (such as the thymine nucleotide) into nucleosides (such as thymidine) and phosphate. The nucleosides, in turn, are subsequently broken down:
- - in the lumen of the digestive system by nucleosidases into nitrogenous bases and ribose (or deoxyribose).
- - inside the cell by nucleoside phosphorylases into nitrogenous bases, and ribose-1-phosphate (or deoxyribose-1-phosphate).
Nucleosides differ from nucleotides by having a hydroxyl group attached to carbon number 5 (the one that isn't in the ring) of the ribose, rather than one or more phosphate groups.
See also
- Nucleobase
- Nucleotide
- RNA
- Adenosine triphosphate (ATP)
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
Template:Biochem-stub Note: Nucleosides can be produced by combining nucleobases with deoxyribose rings as well.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.