Difference between revisions of "Malachite" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 1: | Line 1: | ||
| − | ''This article is about the mineral | + | {{Claimed}} |
| + | :''This article is about the mineral.'' | ||
{{Infobox mineral | {{Infobox mineral | ||
| name = Malachite | | name = Malachite | ||
| Line 6: | Line 7: | ||
| boxbgcolor = | | boxbgcolor = | ||
| image = Malachite Macro 43.jpg | | image = Malachite Macro 43.jpg | ||
| − | | caption = Malachite showing typical radiating | + | | caption = Malachite showing typical radiating fibrous and botryoidal structure. |
| formula = Cu<sub>2</sub>CO<sub>3</sub>(OH)<sub>2</sub> | | formula = Cu<sub>2</sub>CO<sub>3</sub>(OH)<sub>2</sub> | ||
| molweight = | | molweight = | ||
| Line 31: | Line 32: | ||
}} | }} | ||
| − | '''Malachite''' is a [[Carbonate minerals|carbonate mineral]] | + | '''Malachite''' is a [[Carbonate minerals|carbonate mineral]] of [[copper]], with a vibrant green color. It is usually found with copper deposits associated with [[limestone]]. |
| − | + | == Etymology == | |
| − | The | + | The name malachite derives (via [[Latin]] and [[French language|French]]) from the [[Greek language|Greek]] word ''molochitis'', meaning "[[mallow]]-green stone." That, in turn, may be derived from ''molochē'' (variant of ''malachē''), meaning "mallow." |
| − | + | == Occurrence == | |
| − | + | Malachite often results from weathering of [[copper]] [[ore]]s and is often found together with [[azurite]] (Cu<sub>3</sub>(CO<sub>3</sub>)<sub>2</sub>(OH)<sub>2</sub>), [[goethite]], and [[calcite]]. Except for the vibrant green color, the properties of malachite are very similar to those of azurite and aggregates of the two minerals together are frequently found, although malachite is more common than azurite. Typically found with copper deposits associated with [[limestone]], the source of the carbonate. | |
| − | == | + | Large quantities of malachite have been mined in the [[Ural]] mountains of [[Russia]]. In addition, it has been found in the [[Democratic Republic of Congo]]; [[Tsumeb]], [[Namibia]]; [[Mexico]]; [[Broken Hill, New South Wales]], [[Australia]]; [[England]]; and [[Lyon]], [[France]]. It also occurs in the Southwestern [[United States]], especially in [[Arizona]] at [[Bisbee, Arizona|Bisbee]] and [[Morenci, Arizona|Morenci]]. |
| + | |||
| + | In [[Israel]], malachite is extensively mined at [[Timna]], often called [[King Solomon's Mines]]. Archeological evidence indicates that the mineral has been mined and smelted at this site for over 3,000 years. Most of Timna's current production is also smelted, but the finest pieces are worked into silver jewelry. | ||
| + | |||
| + | == Characteristics == | ||
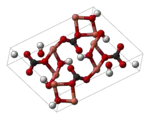
| + | [[Image:Malachite-unit-cell-3D-balls.png|thumb|150px|left|[[Ball-and-stick model]] of a [[unit cell]] malachite.]] | ||
| + | |||
| + | Chemically, malachite is called [[copper(II) carbonate]] [[hydroxide]], with the formula Cu<sub>2</sub>CO<sub>3</sub>(OH)<sub>2</sub>. It crystallizes in the [[monoclinic]] crystal system, and most often forms [[botryoidal]], fibrous, or [[stalagmite|stalagmitic]] masses. Individual crystals are rare, but they do occur as slender to acicular prisms. [[Pseudomorph]]s after more tabular or blocky azurite crystals also occur. | ||
| + | |||
| + | == Uses == | ||
| + | |||
| + | Malachite was used as a mineral pigment in green paints from antiquity until about 1800. The pigment is moderately lightfast, very sensitive to [[acid]]s, and varies in color. The natural form was being replaced by its synthetic form, [[verditer]] amongst other synthetic greens. | ||
| + | |||
| + | It is also used for decorative purposes, such as in the Malachite Room in the [[Hermitage Museum]], which features a huge [[:Image:New hermitage interior.jpg|malachite vase]]. "The [[Tazza]]," one of the largest pieces of malachite in North America and a gift from [[Tsar Nicholas II]], stands as the focal point in the center of the room of [[Linda Hall Library]]. | ||
| + | |||
| + | == Gallery == | ||
<gallery> | <gallery> | ||
Image:Malachite Zaire.jpg|A polished slice of Malachite. | Image:Malachite Zaire.jpg|A polished slice of Malachite. | ||
| − | |||
Image:Azurite with malachite and others.jpg|Malachite crystals atop blue [[azurite]], with brown [[cuprite]] on the white [[kaolinite]] | Image:Azurite with malachite and others.jpg|Malachite crystals atop blue [[azurite]], with brown [[cuprite]] on the white [[kaolinite]] | ||
Image:MoreMalachite.jpg|Malachite from the [[Democratic Republic of Congo]] | Image:MoreMalachite.jpg|Malachite from the [[Democratic Republic of Congo]] | ||
| + | </gallery> | ||
| + | |||
| + | == See also == | ||
| − | + | * [[Azurite]] | |
| + | * [[Gemstone]] | ||
| + | * [[Mineral]] | ||
==References== | ==References== | ||
| − | *Hurlbut, Cornelius S. | + | |
| + | * Hurlbut, Cornelius S., and Cornelis Klein. 1985. ''Manual of Mineralogy''. 20th ed. New York: John Wiley. ISBN 0-471-80580-7 | ||
| + | * Sofianides, Anna S., and George E. Harlow. 1997. ''Gems & Crystals''. London: Parkgate Books. ISBN 1-85585-391-4 | ||
| + | * Weinstein, Michael. 1967. ''The World of Jewel Stones''. New York: Sheridan House. | ||
| + | |||
| + | == External links == | ||
| + | |||
*[http://mineral.galleries.com/minerals/carbonat/malachit/malachit.htm Mineral Galleries] | *[http://mineral.galleries.com/minerals/carbonat/malachit/malachit.htm Mineral Galleries] | ||
*[http://webmineral.com/data/Malachite.shtml Webmineral data] | *[http://webmineral.com/data/Malachite.shtml Webmineral data] | ||
| Line 57: | Line 83: | ||
*[http://www.hermitagemuseum.org/html_En/08/hm88_0_1_62.html Virtual tour of the Malachite Room] | *[http://www.hermitagemuseum.org/html_En/08/hm88_0_1_62.html Virtual tour of the Malachite Room] | ||
| − | + | [[Category:Physical sciences]] | |
| − | + | [[Category:Earth sciences]] | |
| − | + | [[Category:Geology]] | |
| − | [[Category: | + | [[Category:Minerals]] |
| − | [[Category: | ||
| − | [[Category: | ||
| − | + | {{credit|159209446}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 19:58, 24 September 2007
- This article is about the mineral.
| Malachite | |
|---|---|
 Malachite showing typical radiating fibrous and botryoidal structure. |
|
| General | |
| Category | Mineral |
| Chemical formula | Cu2CO3(OH)2 |
| Identification | |
| Color | Green |
| Crystal habit | Massive, botryoidal, stalactitic |
| Crystal system | Monoclinic - prismatic |
| Cleavage | Perfect |
| Fracture | Conchoidal to splintery |
| Mohs Scale hardness | 3.5 - 4 |
| Luster | Dull/vitreous in large quantities, silky in crystal form |
| Streak | green |
| Specific gravity | 3.6 - 4 |
| {{{density}}} | |
Malachite is a carbonate mineral of copper, with a vibrant green color. It is usually found with copper deposits associated with limestone.
Etymology
The name malachite derives (via Latin and French) from the Greek word molochitis, meaning "mallow-green stone." That, in turn, may be derived from molochē (variant of malachē), meaning "mallow."
Occurrence
Malachite often results from weathering of copper ores and is often found together with azurite (Cu3(CO3)2(OH)2), goethite, and calcite. Except for the vibrant green color, the properties of malachite are very similar to those of azurite and aggregates of the two minerals together are frequently found, although malachite is more common than azurite. Typically found with copper deposits associated with limestone, the source of the carbonate.
Large quantities of malachite have been mined in the Ural mountains of Russia. In addition, it has been found in the Democratic Republic of Congo; Tsumeb, Namibia; Mexico; Broken Hill, New South Wales, Australia; England; and Lyon, France. It also occurs in the Southwestern United States, especially in Arizona at Bisbee and Morenci.
In Israel, malachite is extensively mined at Timna, often called King Solomon's Mines. Archeological evidence indicates that the mineral has been mined and smelted at this site for over 3,000 years. Most of Timna's current production is also smelted, but the finest pieces are worked into silver jewelry.
Characteristics
Chemically, malachite is called copper(II) carbonate hydroxide, with the formula Cu2CO3(OH)2. It crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses. Individual crystals are rare, but they do occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur.
Uses
Malachite was used as a mineral pigment in green paints from antiquity until about 1800. The pigment is moderately lightfast, very sensitive to acids, and varies in color. The natural form was being replaced by its synthetic form, verditer amongst other synthetic greens.
It is also used for decorative purposes, such as in the Malachite Room in the Hermitage Museum, which features a huge malachite vase. "The Tazza," one of the largest pieces of malachite in North America and a gift from Tsar Nicholas II, stands as the focal point in the center of the room of Linda Hall Library.
Gallery
Malachite from the Democratic Republic of Congo
See also
ReferencesISBN links support NWE through referral fees
- Hurlbut, Cornelius S., and Cornelis Klein. 1985. Manual of Mineralogy. 20th ed. New York: John Wiley. ISBN 0-471-80580-7
- Sofianides, Anna S., and George E. Harlow. 1997. Gems & Crystals. London: Parkgate Books. ISBN 1-85585-391-4
- Weinstein, Michael. 1967. The World of Jewel Stones. New York: Sheridan House.
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.