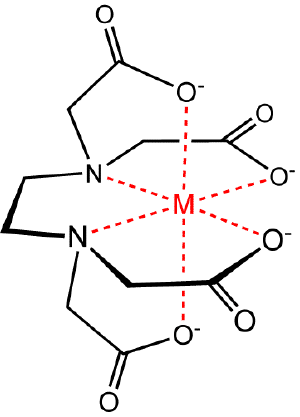
Chelation
Chelation is the binding or complexation of a bi- or multidentate ligand. These ligands, which are often organic compounds, are called chelants, chelators, chelating agents, or sequestering agent. The ligand forms a chelate complex with the substrate. The term is reserved for complexes in which the metal ion is bound to two or more atoms of the chelating agent.
History and etymology
Chelation is from Greek χηλή, chelè, meaning claw; pronounced /kiːˈleɪʃən/. The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or chele (Greek) of the lobster or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings."[1]
The chelate effect
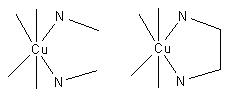
Consider the two equilibria, in aqueous solution, between the copper(II) ion, Cu2+ and ethylenediamine (en) on the one hand and methylamine, MeNH2 on the other.
In (1) the bidentate ligand ethylene diamine forms a chelate complex with the copper ion. Chelation results in the formation of a five–membered ring. In (2) the bidentate ligand is replaced by two monodentate methylamine ligands of approximately the same donor power, meaning that the enthalpy of formation of Cu—N bonds is approximately the same in the two reactions. Under conditions of equal copper concentrations and when then concentration of methylamine is twice the concentration of ethylenediamine, the concentration of the complex (1) will be greater than the concentration of the complex (2). The effect increases with the number of chelate rings so the concentration of the EDTA complex, which has six chelate rings, is much much higher than a corresponding complex with two monodentate nitrogen donor ligands and four monodentate carboxylate ligands. Thus, the phenomenon of the chelate effect is a firmly established empirical fact.
The thermodynamic approach to explaining the chelate effect considers the equilibrium constant for the reaction: the larger the equilibrium constant, the higher the concentration of the complex.
- [Cu(en] =β11[Cu][en]
- [Cu(MeNH2)2]= β12[Cu][MeNH2]2
Electrical charges have been omitted for simplicity of notation. The square brackets indicate concentration, and the subscripts to the stability constants, β, indicate the stoichiometry of the complex. When the analytical concentration of methylamine is twice that of ethylenediamine and the concentration of copper is the same in both reactions, the concentration [Cu(en)] is much higher than the concentration [Cu(MeNH2)2] because β11 >> β12.
An equilibrium constant, K, is related to the standard Gibbs free energy, ΔG![]() by
by
where R is the gas constant and T is the temperature in Kelvin. ΔH![]() is the standard enthalpy change of the reaction and ΔS
is the standard enthalpy change of the reaction and ΔS![]() is the standard entropy change. It has already been posited that the enthalpy term should be approximately the same for the two reactions. Therefore the difference between the two stability constants is due to the entropy term. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.[2]
is the standard entropy change. It has already been posited that the enthalpy term should be approximately the same for the two reactions. Therefore the difference between the two stability constants is due to the entropy term. In equation (1) there are two particles on the left and one on the right, whereas in equation (2) there are three particles on the left and one on the right. This means that less entropy of disorder is lost when the chelate complex is formed than when the complex with monodentate ligands is formed. This is one of the factors contributing to the entropy difference. Other factors include solvation changes and ring formation. Some experimental data to illustrate the effect are shown in the following table.[2]
Equilibrium log β ΔG 
ΔH  /kJ mol−1
/kJ mol−1−TΔS  /kJ mol−1
/kJ mol−1
Cd2+ + 4 MeNH2  Cd(MeNH2)42+
Cd(MeNH2)42+
6.55 -37.4 -57.3 19.9 Cd2+ + 2 en  Cd(en)22+
Cd(en)22+
10.62 -60.67 -56.48 -4.19
These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. In general it is difficult to account precisely for thermodynamic values in terms of changes in solution at the molecular level, but it is clear that the chelate effect is predominantly an effect of entropy.
Other explanations, Including that of Schwarzenbach,[3] are discussed in Greenwood and Earnshaw (loc.cit).
Chelation in nature
Virtually all biochemicals exhibit the ability to dissolve certain metal cations. Thus, proteins, polysaccharides, and polynucleic acids are excellent polydentate ligands for many metal ions. In addition to these adventitious chelators, several biomolecules are produced to specifically bind certain metals (see next section). Histidine, malate and phytochelatin are typical chelators used by plants.[4][5][6]
In biochemistry and microbiology
Virtually all metalloenzymes feature metals that are chelated, usually to peptides or cofactors and prosthetic groups.[7] Such chelating agents include the porphyrin rings in hemoglobin and chlorophyll. Many microbial species produce water-soluble pigments that serve as chelating agents, termed siderophores. For example, species of Pseudomonas are known to secrete pycocyanin and pyoverdin that bind iron. Enterobactin, produced by E. coli, is the strongest chelating agent known.
In geology
In earth science, chemical weathering is attributed to organic chelating agents, e.g. peptides and sugars, that extract metal ions from minerals and rocks.[8] Most metal complexes in the environment and in nature are bound in some form of chelate ring, e.g. with "humic acid" or a protein. Thus, metal chelates are relevant to the mobilization of metals in the soil, the uptake and the accumulation of metals into plants and micro-organisms. Selective chelation of heavy metals is relevant to bioremediation, e.g. removal of 137Cs from radioactive waste.[9]
Applications
Chelators are used in chemical analysis, as water softeners, and are ingredients in many commercial products such as shampoos and food preservatives. Citric acid is used to soften water in soaps and laundry detergents. A common synthetic chelator is EDTA. Phosphonates are also well known chelating agents. Chelators are used in water treatment programs and specifically in steam engineering, e.g., boiler water treatment system: Chelant Water Treatment system.
Heavy metal detoxification
Chelation therapy is the use of chelating agents to detoxify poisonous metal agents such as mercury, arsenic, and lead by converting them to a chemically inert form that can be excreted without further interaction with the body, and has been approved by the U.S. FDA in 1991. Chelation is also used but unproven as a treatment for autism.[10]
In addition to being beneficial, chelating agents can also be dangerous. The U.S. CDC reports that use of Na2EDTA instead of CaEDTA has resulted in fatalities due to hypocalcemia.[11]
Other medical applications
Antibiotic drugs of the tetracycline family are chelators of Ca2+ and Mg2+ ions.
EDTA is also used in root canal treatment as a way to irrigate the canal. EDTA softens the dentin facilitating access to the entire canal length and to remove the smear layer formed during instrumentation.
Gadolinium(III) compounds and chelates are often used as contrast agents in MRI scans.
See also
Notes
- ↑ Morgan, Gilbert T.; Drew, Harry D. K. (1920). CLXII.—Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. J. Chem. Soc., Trans. 117: 1456.
- ↑ Greenwood, N. N.; & Earnshaw, A. 1997. Chemistry of the Elements (2nd Edn.). Oxford, UK: Butterworth-Heinemann. ISBN 0-7506-3365-4. p 910
- ↑ Schwarzenbach, G (1952). Der Chelateffekt. Helv. Chim. Acta 35: 2344–2359.
- ↑ U Krämer, J D Cotter-Howells, J M Charnock, A H J M Baker, J A C Smith (1996). Free histidine as a metal chelator in plants that accumulate nickel. Nature 379: 635–638.
- ↑ Jurandir Vieira Magalhaes (2006). Aluminum tolerance genes are conserved between monocots and dicots. Proc Natl Acad Sci USA 103 (26): 9749.
- ↑ Suk-Bong Ha, Aaron P. Smith, Ross Howden, Wendy M. Dietrich, Sarah Bugg, Matthew J. O'Connell, Peter B. Goldsbrough, and Christopher S. Cobbett (1999). Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1164.
- ↑ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ↑ Dr. Michael Pidwirny, University of British Columbia Okanagan, http://www.physicalgeography.net/fundamentals/10r.html
- ↑ Prasad (ed). Metals in the Environment. University of Hyderabad. Dekker, New York, 2001
- ↑ Doja A, Roberts W (2006). Immunizations and autism: a review of the literature. Can J Neurol Sci 33 (4): 341–46.
- ↑ U.S. Centers for Disease Control, "Deaths Associated with Hypocalcemia from Chelation Therapy" (March 3, 2006), http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5508a3.htm
ReferencesISBN links support NWE through referral fees
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.