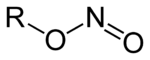Difference between revisions of "Nitrite" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
(claimed) |
||
| Line 1: | Line 1: | ||
| + | {{Claimed}} | ||
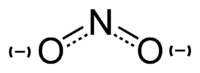
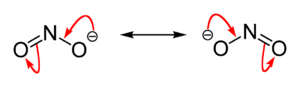
[[Image:Nitrite-ion-resonance-hybrid.png|thumb|200px|right|A [[Resonance (chemistry)#Resonance as a diagrammatic tool|resonance hybrid]], showing the N-O bonds in the nitrite ion have a [[bond order]] of about 1.5, leaving most of the single negative charge shared between the terminal oxygen atoms]] | [[Image:Nitrite-ion-resonance-hybrid.png|thumb|200px|right|A [[Resonance (chemistry)#Resonance as a diagrammatic tool|resonance hybrid]], showing the N-O bonds in the nitrite ion have a [[bond order]] of about 1.5, leaving most of the single negative charge shared between the terminal oxygen atoms]] | ||
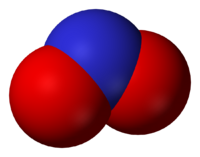
[[Image:Nitrite-3D-vdW.png|thumb|200px|right|[[Space-filling model]] of NO<sub>2</sub><sup>−</sup>]] | [[Image:Nitrite-3D-vdW.png|thumb|200px|right|[[Space-filling model]] of NO<sub>2</sub><sup>−</sup>]] | ||
| Line 32: | Line 33: | ||
Nitrites should be confused neither with [[nitrate]]s, the salts of [[nitric acid]], nor with [[nitro compound]]s, though they share the formula RNO<sub>2</sub>. The nitrite [[anion]] NO<sub>2</sub><sup>−</sup> should not be confused with the [[nitronium]] [[cation]] NO<sub>2</sub><sup>+</sup>. | Nitrites should be confused neither with [[nitrate]]s, the salts of [[nitric acid]], nor with [[nitro compound]]s, though they share the formula RNO<sub>2</sub>. The nitrite [[anion]] NO<sub>2</sub><sup>−</sup> should not be confused with the [[nitronium]] [[cation]] NO<sub>2</sub><sup>+</sup>. | ||
| − | == | + | == Notes == |
| − | + | <references/> | |
==References== | ==References== | ||
| − | |||
| − | |||
| + | ==External links== | ||
| + | * [http://www.atsdr.cdc.gov/HEC/CSEM/nitrate/ Case Studies in Environmental Medicine - Nitrate/Nitrite Toxicity] | ||
| − | [[Category: | + | [[Category:Physical sciences]] |
| − | [[Category: | + | [[Category:Chemistry]] |
| − | |||
| − | |||
| − | + | {{credit|113495185}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 17:23, 9 March 2007
The nitrite ion is NO2−. The anion is bent, being isoelectronic with O3.
A nitrite is either a salt or an ester of nitrous acid.
Examples
- nitrous acid, HNO2
- sodium nitrite, NaNO2
- methyl nitrite, CH3NO2
- alkyl nitrites, commonly known as poppers
See category for a bigger list.
Inorganic nitrites
In inorganic chemistry, nitrites are salts of nitrous acid HNO2. They contain the nitrite ion NO2−. Nitrites of the alkali and alkaline earth metals can be synthesized by reacting a mixture of nitrogen monoxide NO and nitrogen dioxide NO2 with the corresponding metal hydroxide solution, as well as through the thermal decomposition of the corresponding nitrate. Other nitrites are available through the reduction of the corresponding nitrates.
Sodium nitrite is used for the "curing of meat" because it prevents bacterial growth and, in a reaction with the meat's myoglobin, gives the product a desirable dark red color. Because of the toxicity of nitrite (lethal dose of nitrite for humans is about 22 mg per kg body weight), the maximum allowed nitrite concentration in meat products is 200 ppm. Under certain conditions, especially during cooking, nitrites in meat can react with degradation products of amino acids, forming nitrosamines, which are known carcinogens.
Nitrite is detected and analyzed by the Griess Reaction, involving the formation of a deeply red-color azo dye upon treatment of a NO2−-containing sample with sulfanilic acid and naphthyl-1-amine in the presence of acid.[1]
Nitrite can be reduced to nitric oxide or ammonia by many species of bacteria.
Organic nitrites
In organic chemistry, nitrites are esters of nitrous acid and contain the nitrosooxy functional group. They possess the general formula RONO, where R is an aryl or alkyl group. Amyl nitrite is used in medicine for the treatment of heart diseases.
Nitrites should be confused neither with nitrates, the salts of nitric acid, nor with nitro compounds, though they share the formula RNO2. The nitrite anion NO2− should not be confused with the nitronium cation NO2+.
Notes
- ↑ The 125th Anniversary of the Griess Reagent by V. M. Ivanov in Journal of Analytical Chemistry, Vol. 59, No. 10, 2004, pp. 1002–1005. Translated from Zhurnal Analiticheskoi Khimii, Vol. 59, No. 10, 2004, pp. 1109–1112.
ReferencesISBN links support NWE through referral fees
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.