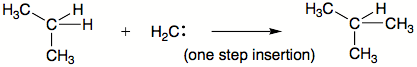Carbene
- Not to be confused with carbine.
In chemistry, a carbene is a highly reactive organic compound with the general molecular formula "R1R2C:." This formula indicates that each molecule has a carbon atom (C) attached to two substituents (R1 and R2), and this carbon atom has two additional (valence) electrons in its outermost shell that account for the molecule's high reactivity. Most carbenes are very short lived, but some persistent carbenes are also known. They can be stabilized in the form of organometallic complexes.
The prototypical carbene is H2C:, also called methylene. One well-studied carbene is Cl2C:, or dichlorocarbene, which can be generated in situ by the reaction of chloroform with a strong base.
Structure
Generally, there are two types of carbenes, known as singlet and triplet carbenes.[1] They differ in structure based on the distribution of electrons in orbitals of the reactive carbon atom.
- In a singlet carbene, the reactive carbon atom has three sp2 hybrid orbitals, with a pair of electrons occupying one of these orbitals. In addition, it has one empty p orbital crossing the plane containing R1, R2, and the free electron pair (as shown in the diagram on the right).
- In a triplet carbene, the reactive carbon has two unpaired electrons distributed in one of two possible orbital configurations: (a) The reactive carbon has three sp2 hybrid orbitals and one unhybridized p orbital. One unpaired electron occupies an sp2 hybrid orbital and the other occupies a p orbital. (b) The reactive carbon atom has two sp hybrid orbitals (in a linear structure) and two unhybridized p orbitals. The two unpaired electrons occupy the latter two p orbitals (as shown in the diagram).
Most carbenes have a nonlinear triplet ground state, except for those with nitrogen, oxygen, or sulfur atoms, and dihalocarbenes.
Carbenes are called singlet or triplet depending on the electronic spins they possess. Triplet carbenes are paramagnetic and may be observed by electron paramagnetic resonance spectroscopy (EPR) if they persist long enough. The total spin of singlet carbenes is zero while that of triplet carbenes is one (in units of ). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (as determined by EPR). Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
For simple hydrocarbons, triplet carbenes usually have energies 8 kcal/mol (33 kJ/mol) lower than singlet carbenes. Thus, in general, triplet is the more stable state (the ground state) and singlet is the excited state species. Substituents that can donate electron pairs may stabilize the singlet state by delocalizing the pair into an empty p-orbital. If the energy of the singlet state is sufficiently reduced, it will actually become the ground state.
No viable strategies exist for triplet stabilization. The carbene called 9-fluorenylidene has been shown to be a rapidly equilibrating mixture of singlet and triplet states with an approximately 1.1 kcal/mol (4.6 kJ/mol) energy difference.[2] It is, however, debatable whether diaryl carbenes such as the fluorene carbene are true carbenes because the electrons can delocalize to such an extent that they become in fact biradicals. In silico experiments suggest that triplet carbenes can be stabilized with electropositive groups such as trifluorosilyl groups.[3]
Reactivity
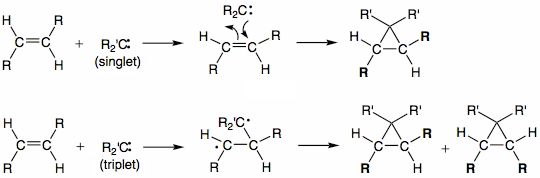
Singlet and triplet carbenes do not demonstrate the same reactivity. Singlet carbenes generally participate in cheletropic reactions as either electrophiles or nucleophiles. Singlet carbene with its unfilled p-orbital should be electrophilic. Triplet carbenes should be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate with two unpaired electrons whereas singlet carbene can react in a single concerted step. Addition of singlet carbenes to olefinic double bonds is more stereoselective than that of triplet carbenes. Addition reactions with alkenes can be used to determine whether the singlet or triplet carbene is involved.
Reactions of singlet methylene are stereospecific while those of triplet methylene are not. For instance the reaction of methylene generated from photolysis of diazomethane with cis-2-butene and trans-2-butene is stereospecific which proves that in this reaction methylene is a singlet.[4]
Reactivity of a particular carbene depends on the substituent groups, preparation method, reaction conditions such as presence or absence of metals. Some of the reactions carbenes can do are insertions into C-H bonds, skeletal rearrangements, and additions to double bonds. Carbenes can be classified as nucleophilic, electrophilic, or ambiphilic. Reactivity is especially strongly influenced by substituents. For example, if a substituent is able to donate a pair of electrons, most likely carbene will not be electrophilic. Alkyl carbenes insert much more selectively than methylene, which does not differentiate between primary, secondary, and tertiary C-H bonds.
Carbenes add to double bonds to form cyclopropanes. A concerted mechanism is available for singlet carbenes. Triplet carbenes do not retain stereochemistry in the product molecule. Addition reactions are commonly very fast and exothermic. The slow step in most instances is generation of carbene. A well-known reagent employed for alkene-to-cyclopropane reactions is Simmons-Smith reagent. It is a system that includes copper, zinc, and iodine, where the active reagent is believed to be iodomethylzinc iodide.
Carbenes are also involved in insertion reactions, in which the carbene interposes itself into an existing bond. The order of preference is commonly: (1) X-H bonds, where X is not carbon; (2) C-H bond, and (3) C-C bond. Insertions may or may not occur in single step.
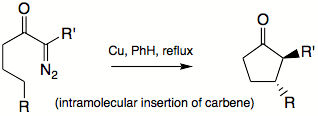
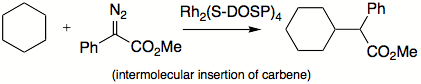
Intramolecular insertion reactions present new synthetic solutions. Generally, rigid structures favor such insertions to happen. When an intramolecular insertion is possible, no intermolecular insertions are seen. In flexible structures, five-membered ring formation is preferred to six-membered ring formation. Both inter- and intramolecular insertions are amendable to asymmetric induction by choosing chiral ligands on metal centers.
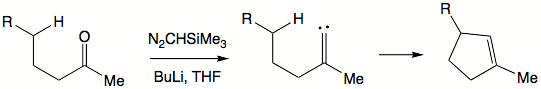
Alkylidene carbenes are alluring in that they offer formation of cyclopentene moieties. To generate an alkylidene carbene a ketone can be exposed to trimethylsilyl diazomethane.
Generation of carbenes
Carbenes may be produced by a number of different reactions, some of which are noted below.
- Most commonly, photolytic, thermal, or transition metal catalyzed decomposition of diazoalkanes is used to create carbene molecules. A variation on catalyzed decomposition of diazoalkanes is the Bamford-Stevens reaction, which gives carbenes in aprotic solvents and carbenium ions in protic solvents.
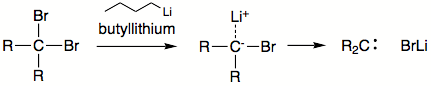
- Another method is induced elimination of halogen from gem-dihalides or HX from CHX3 moiety, employing organolithium reagents (or another strong base). It is not certain that in these reactions actual free carbenes are formed. In some cases there is evidence that completely free carbene is never present. It is likely that instead a metal-carbene complex forms. Nevertheless, these metallocarbenes (or carbenoids) give the expected products.
- Photolysis of diazirines and epoxides can also be employed. Diazirines contain 3-membered rings and are cyclic forms of diazoalkanes. The strain of the small ring makes photoexcitation easy. Photolysis of epoxides gives carbonyl compounds as side products. With asymmetric epoxides, two different carbonyl compounds can potentially form. The nature of substituents usually favors formation of one over the other. One of the C-O bonds will have a greater double bond character and thus will be stronger and less likely to break. Resonance structures can be drawn to determine which part will contribute more to the formation of carbonyl. When one substituent is alkyl and another aryl, the aryl-substituted carbon is usually released as a carbene fragment.
- Thermolysis of alpha-halomercury compounds is another method to generate carbenes.
- Rhodium and copper complexes promote carbene formation.
- Carbenes are intermediates in the Wolff rearrangement.
Stabilization of carbenes and carbene ligands
Carbenes can be stabilized as organometallic species. These transition metal carbene complexes fall into the following three categories, of which the first two are the most clearly defined:
- Fischer carbenes, in which the carbene is tethered to a metal that bears an electron-withdrawing group (usually a carbonyl).
- Schrock carbenes, in which the carbene is tethered to a metal that bears an electron-donating group.
- Persistent carbenes, also known as stable carbenes or Arduengo carbenes. They include the class of N-heterocyclic carbenes (NHCs) and are often are used as ancillary ligands in organometallic chemistry.
An additional group of carbenes, known as foiled carbenes, derive their stability from the proximity of a double bond—that is, their ability to form conjugated systems.
See also
Notes
- ↑ Robert T. Morrison and Robert N. Boyd, Organic Chemistry, 6th ed. (Englewood Cliffs, NJ: Prentice Hall, 1992, ISBN 0136436692).
- ↑ Peter B. Grasse, Beth-Ellen Brauer, Joseph J. Zupancic, Kenneth J. Kaufmann, and Gary B. Schuster, Chemical and Physical Properties of Fluorenylidene: Equilibrium of the Singlet and Triplet Carbenes, J. Am. Chem. Soc. 105 (1983): 6833-6845.
- ↑ Adelina Nemirowski and Peter R. Schreiner, Electronic Stabilization of Ground State Triplet Carbenes, J. Org. Chem. 72 (2007): 9533-9540.
- ↑ Philip S. Skell and Robert C. Woodworth, Structure of Carbene CH2, J. Am. Chem. Soc. 78 (17) (1956): 4496-4497.
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692.
- Solomons, T.W. Graham, and Craig B. Fryhle 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.