Difference between revisions of "Tyrosine" - New World Encyclopedia
(New page: {{Claimed}}) |
|||
| Line 1: | Line 1: | ||
{{Claimed}} | {{Claimed}} | ||
| + | <div> | ||
| + | <!-- Here is a table of data; skip past it to edit the text. —> | ||
| + | <!-- Submit {{:subst:chembox_simple_organic}} to get this template or go to [[:Template:Chembox_simple_organic]]. —> | ||
| + | {| id="bioChemInfoBox" align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090; width: 320px;" | ||
| + | ! {{chembox header}}| '''Tyrosine''' | ||
| + | |- | ||
| + | | align="center" colspan="2" | [[Image:L-tyrosine-skeletal.png|150px|Chemical structure of Tyrosine]][[Image:L-tyrosine-3D-sticks.png|120px|Chemical structure of {{PAGENAME}}]] | ||
| + | |- | ||
| + | | style="width: 30%;" | [[IUPAC nomenclature|Systematic name]] | ||
| + | | (''S'')-2-Amino-3-(4-hydroxy-<br />phenyl)-propanoic acid | ||
| + | |- | ||
| + | | Abbreviations | ||
| + | | '''Tyr<br/>Y''' | ||
| + | |- | ||
| + | | [[Chemical formula]] | ||
| + | | C<sub>9</sub>H<sub>11</sub>NO<sub>3</sub> | ||
| + | |- | ||
| + | | [[Molecular mass]] | ||
| + | | 181.19 g mol<sup>-1</sup> | ||
| + | |- | ||
| + | | [[Melting point]] | ||
| + | | 343 °C | ||
| + | |- | ||
| + | | [[Density]] | ||
| + | | 1.456 g cm<sup>-3</sup> | ||
| + | |- | ||
| + | | [[Isoelectric point]] | ||
| + | | 5.66 | ||
| + | |- | ||
| + | | [[Acid dissociation constant|p''K''<sub>a</sub>]] | ||
| + | | 2.24<br/>9.04<br/>10.10 | ||
| + | |- | ||
| + | | [[Molar extinction coefficient]] | ||
| + | | 1420 M<sup>-1</sup> cm<sup>-1</sup> at 274.6 nm | ||
| + | |- | ||
| + | | [[PubChem]] | ||
| + | | [http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1153 1153] | ||
| + | |- | ||
| + | | [[CAS registry number|CAS number]] | ||
| + | | [60-18-4] | ||
| + | |- | ||
| + | | [[EINECS number]] | ||
| + | | 200-460-4 | ||
| + | |- | ||
| + | | [[Simplified molecular input line entry specification|SMILES]] | ||
| + | | {{SMILES|S=N[C@@H](Cc1ccc(O)cc1)C(O)=O}} | ||
| + | |- | ||
| + | | align="center" colspan="2" bgcolor="#ffffff" | '''[[absorption spectrum|Absorption]] and [[emission spectrum]]'''<br/>[[Image:Tyrosine abs flu.png|290px|Absorption (blue) and fluorescence (red) of tyrosine in water (pH 7)]] | ||
| + | Absorbance and fluorescence of tyrosine in water/buffer | ||
| + | |- | ||
| + | | {{chembox header}} | <small>[[wikipedia:Chemical infobox|Disclaimer and references]]</small> | ||
| + | |- | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | '''Tyrosine''' (from the [[Greek language|Greek]] ''tyros'', meaning ''cheese'', as it was first discovered in 1846 by German chemist [[Justus von Liebig]] in cheese<ref>[http://www.infoplease.com/ce6/sci/A0849873.html Tyrosine at infoplease.com]</ref><ref>[http://www.etymonline.com/index.php?l=t&p=24 Tyrosine at etymonline.com]</ref>), '''4-hydroxyphenylalanine''', or '''2-amino-3(4-hydroxyphenyl)-propanoic acid''', is one of the 20 [[amino acid]]s that are used by [[cell (biology)|cells]] to [[protein biosynthesis|synthesize]] [[protein]]s. It has a [[phenol]] [[side chain]] with a [[hydroxyl]] group. Upon the location of the hydroxyl group, there are three structural isomers of Tyr, namely para-Tyr (p-Tyr), meta-Tyr (m-Tyr) and ortho-Tyr (o-Tyr). Enzymatically, only the first isomer (p-Tyr) is produced from L-Phe by the Phe-hydroxylase enzyme. The other two isoforms, m-Tyr and o-Tyr can be produced as a consequence of [[free radical]] attack on Phe in states with increased [[oxidative stress]]. | ||
| + | |||
| + | [[Image:Phe_Tyr.jpg|left|270px|tyrosine molecule]] | ||
| + | |||
| + | Tyrosine is converted to [[levodopa]] by [[tyrosine hydroxylase]], an enzyme. | ||
| + | |||
| + | Some of the tyrosine residues can be ''tagged'' with a phosphate group ([[phosphorylation|phosphorylated]]) by [[protein kinase]]s. (In its phosphorylated state, it is referred to as '''phosphotyrosine'''.). Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as [[tyrosine sulfation]]. [[Tyrosine sulfation]] is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine. | ||
| + | Tyrosine is also precursor to the [[thyroid]] [[hormone]]s ''[[thyroxine]]'' and ''[[triiodothyronine]]'', the pigment ''[[melanin]]'', and the biologically-active [[catecholamine]]s ''[[dopamine]]'', ''[[norepinephrine]]'' and ''[[epinephrine]]''. | ||
| + | |||
| + | In [[Papaver somniferum]], the opium poppy, it is used to produce [[morphine]]. | ||
| + | __NOTOC__ | ||
| + | == Biosynthesis == | ||
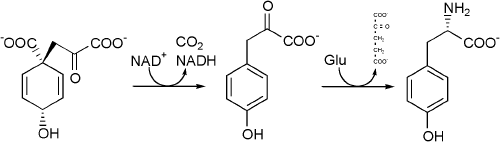
| + | Tyrosine cannot be completely synthesized by animals, although it can be made by hydroxylation of [[phenylalanine]] if the latter is in abundant supply. It is produced by plants and most microorganisms from [[Prephenic acid|prephenate]], an intermediate on the [[Shikimic acid|shikimate pathway]]. | ||
| + | |||
| + | Prephenate is [[Oxidation|oxidatively]] [[Decarboxylation|decarboxylated]] with retention of the [[hydroxyl]] group to give ''p''-hydroxyphenylpyruvate. This is [[Transamination|transaminated]] using [[Glutamic acid|glutamate]] as the nitrogen source to give tyrosine and [[Ketoglutaric acid|α-ketoglutarate]]. | ||
| + | |||
| + | [[Image:Tyrosine biosynthesis.png|500px]] | ||
| + | |||
| + | ==='''Tyrosine hydroxylase'''=== | ||
| + | [[Tyrosine hydroxylase]] (TH) is the rate-limiting [[enzyme]] involved in the synthesis of the [[catecholamines]] such as [[dopamine]], [[norepinephrine]] and [[epinephrine]]. | ||
| + | |||
| + | == Medical use == | ||
| + | L-Tyrosine is sometimes recommended by practitioners as helpful for [[weight loss]], [[clinical depression]], [[Parkinson's Disease]], and [[phenylketonuria]]; however, one study found that it had no impact on endurance exercise performance. <ref>Parcell A.C., et al. Effects of L-tyrosine and carbohydrate ingestion on performance. Journal of Applied Physiology 2002 Nov; 93(5): 1590-97. [http://jap.physiology.org/cgi/content/abstract/93/5/1590 Abstract]</ref> | ||
| + | |||
| + | == See also == | ||
| + | * [[Tyramine]] | ||
| + | * [[Alkaptonuria]] | ||
| + | * [[Tyrosinemia]] | ||
| + | * [[Albinism]] | ||
| + | * [[Tyrosine sulfation]] | ||
| + | |||
| + | ==References== | ||
| + | *AJ Hoffhines et al. ''Journal of Biological Chemistry'' 281:37877-37887, 2006[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17046811&itool=iconpmc&query_hl=1&itool=pubmed_docsum] | ||
| + | *GA Molnar et al. ''Kidney International'' 68:2281-2287, 2005 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16221230&query_hl=1&itool=pubmed_docsum Abstract] | ||
| + | *GA Molnar et al. ''Free Radical Research'' 39(12):1359-1366, 2005 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16298866&query_hl=1&itool=pubmed_docsum Abstract] | ||
| + | |||
| + | ==Notes== | ||
| + | <references/> | ||
| + | |||
| + | == External links == | ||
| + | *[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/PheTyr.html Phenylalanine and tyrosine biosynthesis] | ||
| + | *[http://www.compchemwiki.org/index.php?title=Tyrosine Computational Chemistry Wiki] | ||
| + | *[http://www.exactantigen.com/review/phosphotyrosine.html Phosphotyrosine Antibody] | ||
| + | {{ChemicalSources}} | ||
| + | |||
| + | {{AminoAcids}} | ||
| + | |||
| + | [[Category:Proteinogenic amino acids]] | ||
| + | [[Category:Glucogenic amino acids]] | ||
| + | [[Category:Ketogenic amino acids]] | ||
| + | [[Category:Aromatic amino acids]] | ||
| + | [[Category:Phenols]] | ||
| + | {{credit|136124506}} | ||
| + | [[Category:Life sciences]] | ||
Revision as of 15:46, 15 June 2007
| Tyrosine | |
|---|---|
| |
| Systematic name | (S)-2-Amino-3-(4-hydroxy- phenyl)-propanoic acid |
| Abbreviations | Tyr Y |
| Chemical formula | C9H11NO3 |
| Molecular mass | 181.19 g mol-1 |
| Melting point | 343 °C |
| Density | 1.456 g cm-3 |
| Isoelectric point | 5.66 |
| pKa | 2.24 9.04 10.10 |
| Molar extinction coefficient | 1420 M-1 cm-1 at 274.6 nm |
| PubChem | 1153 |
| CAS number | [60-18-4] |
| EINECS number | 200-460-4 |
| SMILES | N[C@@H](Cc1ccc(O)cc1)C(O)=O |
Absorption and emission spectrum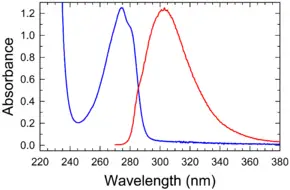
Absorbance and fluorescence of tyrosine in water/buffer | |
| Disclaimer and references | |
Tyrosine (from the Greek tyros, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in cheese[1][2]), 4-hydroxyphenylalanine, or 2-amino-3(4-hydroxyphenyl)-propanoic acid, is one of the 20 amino acids that are used by cells to synthesize proteins. It has a phenol side chain with a hydroxyl group. Upon the location of the hydroxyl group, there are three structural isomers of Tyr, namely para-Tyr (p-Tyr), meta-Tyr (m-Tyr) and ortho-Tyr (o-Tyr). Enzymatically, only the first isomer (p-Tyr) is produced from L-Phe by the Phe-hydroxylase enzyme. The other two isoforms, m-Tyr and o-Tyr can be produced as a consequence of free radical attack on Phe in states with increased oxidative stress.
Tyrosine is converted to levodopa by tyrosine hydroxylase, an enzyme.
Some of the tyrosine residues can be tagged with a phosphate group (phosphorylated) by protein kinases. (In its phosphorylated state, it is referred to as phosphotyrosine.). Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as tyrosine sulfation. Tyrosine sulfation is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine. Tyrosine is also precursor to the thyroid hormones thyroxine and triiodothyronine, the pigment melanin, and the biologically-active catecholamines dopamine, norepinephrine and epinephrine.
In Papaver somniferum, the opium poppy, it is used to produce morphine.
Biosynthesis
Tyrosine cannot be completely synthesized by animals, although it can be made by hydroxylation of phenylalanine if the latter is in abundant supply. It is produced by plants and most microorganisms from prephenate, an intermediate on the shikimate pathway.
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate. This is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
Tyrosine hydroxylase
Tyrosine hydroxylase (TH) is the rate-limiting enzyme involved in the synthesis of the catecholamines such as dopamine, norepinephrine and epinephrine.
Medical use
L-Tyrosine is sometimes recommended by practitioners as helpful for weight loss, clinical depression, Parkinson's Disease, and phenylketonuria; however, one study found that it had no impact on endurance exercise performance. [3]
See also
- Tyramine
- Alkaptonuria
- Tyrosinemia
- Albinism
- Tyrosine sulfation
ReferencesISBN links support NWE through referral fees
- AJ Hoffhines et al. Journal of Biological Chemistry 281:37877-37887, 2006[1]
- GA Molnar et al. Kidney International 68:2281-2287, 2005 Abstract
- GA Molnar et al. Free Radical Research 39(12):1359-1366, 2005 Abstract
Notes
- ↑ Tyrosine at infoplease.com
- ↑ Tyrosine at etymonline.com
- ↑ Parcell A.C., et al. Effects of L-tyrosine and carbohydrate ingestion on performance. Journal of Applied Physiology 2002 Nov; 93(5): 1590-97. Abstract
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.