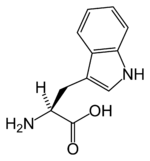
Tryptophan
| Tryptophan | |
|---|---|
| |
| Systematic name | (S)-2-Amino-3-(1H-indol-3-yl)- propionic acid |
| Abbreviations | Trp W |
| Chemical formula | C11H12N2O2 |
| Molecular mass | 204.23 g mol−1 |
| Melting point | 289 °C |
| Density | 1.34 g cm-3 (solid) |
| Isoelectric point | 5.89 |
| pKa | 2.38 9.34 |
| PubChem | 6305 |
| CAS number | [73-22-3] |
| EINECS number | 200-795-6 |
| SMILES | N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |
| Disclaimer and references | |
Tryptophan is an α-amino acid that is found in most proteins and has an indole functional group as a distinguishing structural characteristic. It is a precursor to the neurotransmitter serotonin and the vitamin niacin (also known as nicotinic acid or vitamin B3).
In humans, the L-isomer of tryptophan, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Tryptophan is also classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet. Tryptophan, tyrosine, and phenylalanine are the biggest of the standard amino acids.
Tryptophan's three letter code is Trp, its one letter code is W, its codon is UGG, and its systematic name is 2-Amino-3-(lH-indol-3-yl)-propanoic acid (IUPAC-IUB 1983).
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In tryptophan, only the L-stereoisomer is involved in synthesis of mammalian proteins. However, the D-stereoisomer is occasionally found in natural materials, such as the marine venom peptide contryphan (Pallaghy et al. 1999).
Tyrptophan's chemical formula is C11H12N2O2.
Tryptophan behaves similarly to phenylalanine and tyrosine. Like these two amino acids, tyrptophan contains a large rigid aromatic group on the side chain. Tyrptophan, tyrosine, and phenylalanine—like isoleucine, leucine, and valine—are hydrophobic and tend to orient towards the interior of the folded protein molecule.
Fluorescence
The fluorescence of a folded protein is a mixture of the fluorescence from individual aromatic residues. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of tryptophan residues, with some emissions due to tyrosine and phenylalanine.
Typically, tryptophan has a wavelength of maximum absorption of 280 nm and a wavelength of maximum fluorescence emission of 350 nm. However these fluorescence parameters are strongly dependent on the environment that the tryptophan residue is in, for example the degree of solvent exposure (Brooks 2007). Hence protein fluorescence may be used as a diagnostic of the conformational state a protein (Vivian 2006).
Furthermore, tryptophan fluorescence is strongly influenced by the proximity of other residues (i.e., nearby protonated acidic groups such as aspartic acid or glutamic acid can cause quenching of Trp fluorescence). In addition, tryptophan is a relatively rare amino acid, therefore many proteins contain only one or a few tryptophan residues. Thus, tryptophan fluorescence is a very sensitive measurement of the conformational state of individual tryptophan residues.
Dietary sources
As an essential amino acid, leucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. forutnately, it is found in most proteins, albeit in small amounts.
Major sources include legumes, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, chocolate, oats, bananas, dried dates, sesame, chickpeas, sunflower seeds, pumpkin seeds, spirulina, and peanuts.
Function
The principle function of amino acids, including tryptophan, are as building blocks in protein biosynthesis.
In addition, tryptophan functions as a biochemical precursor for the production of the neurotransmitter serotonin (via [[tryptophan hydroxylase]) and the vitamin niacin (with kynurenine as an intermediate). Serotinin, in turn, can be converted to melatonin (a neurohormone), via 5-hydroxyindole-O-methyltransferase
In organisms which synthesize tryptophan (plants and microorganisms), high levels of this amino acid activate a repressor protein, which in turn binds to the trp operon. Binding of this repressor to its operon prevents transcription of downstream DNA that codes for enzymes involved in the biosynthesis of tryptophan. Hence, high levels of tryptophan prevent additional tryptophan synthesis through a negative feedback loop. Conversely, if the cell's tryptophan level drops, transcription of the operon's genes resumes. This is one example of how gene expression responds rapidly to changes in the cell's internal and external environment.
Use as a dietary supplement
For some time, tryptophan was available in health food stores as a dietary supplement. Since 2002, L-Tryptophan has been sold again in its original form. Many people found tryptophan to be a safe and reasonably effective sleep aid, probably due to its ability to increase brain levels of serotonin (a calming neurotransmitter when present in moderate levels) and/or melatonin (a sleep-inducing hormone secreted by the pineal gland in response to darkness or low light levels).[2]
Clinical research tended to confirm tryptophan's effectiveness as a natural sleep aid and for a growing variety of other conditions typically associated with low serotonin levels or activity in the brain. (Particularly work by Dr. Richard Wurtman at MIT). In particular, tryptophan showed considerable promise as an antidepressant alone, and as an "augmenter" of antidepressant drugs. Other promising indications included relief of chronic pain and reduction of impulsive, violent, manic, addictive, anxiety-related, obsessive, and compulsive behaviours and disorders.
In 1989, a large outbreak of a new, disabling, and in some cases deadly autoimmune illness called eosinophilia-myalgia syndrome (EMS) was traced to one source of L-tryptophan. The bacterial culture used to synthesize tryptophan by a major Japanese manufacturer, Showa Denko KK, had been genetically modified several times to increase tryptophan production during the 1980s.[3]
Along with the higher tryptophan concentrations in the modified culture media, the purification process had also been streamlined to reduce costs, and a purification step that used charcoal absorption to remove some impurities had been omitted.[4] The manufacturer maintained that this process modification allowed another bacterial metabolite through the purification, resulting in the presence of an end-product contaminant responsible for the toxic effects. As of 1996, Showa Denko had destroyed all modified organisms without FDA receipt of culture samples.[5][6] The FDA was unable to publicly establish with certainty what contaminant was the cause of the outbreak.
Most tryptophan was banned from sale in the US in 1991, and other countries followed suit. Tryptophan from one manufacturer, of six, continued to be sold for manufacture of baby formulas. A Rutgers Law Journal article observed, “Political pressures have played a role in the FDA’s decision to ban L-tryptophan as well as its desire to increase its regulatory power over dietary supplements.”[7]
At the time of the ban the FDA did not know, or did not indicate, that EMS was caused by a contaminated batch,[8][9] and yet even when the contamination was discovered and the purification process fixed, the FDA maintained that L-tryptophan was unsafe. In February 2001 the FDA loosened the restrictions on marketing (though not on importation), but still expressed the following concern:
- "Based on the scientific evidence that is available at the present time, we cannot determine with certainty that the occurrence of EMS in susceptible persons consuming L-tryptophan supplements derives from the content of L-tryptophan, an impurity contained in the L-tryptophan, or a combination of the two in association with other, as yet unknown, external factors."[10]
Turkey meat and drowsiness
According to popular belief, eating tryptophan in turkey meat causes drowsiness. Turkey does contain tryptophan,
which does have a documented sleep-inducing effect as it is readily converted into serotonin by the body. However, ingestion of turkey alone has not been proven to have this effect. An additional hypothetical mechanism is as follows: A large quantity of any food, such as a Thanksgiving feast, introduces large quantities of both carbohydrates and branched-chain amino acids releasing insulin. Insulin stimulates the uptake of large neutral branched-chain amino acids (and not tryptophan) by muscle cells through the myocyte membranes. The result is an increase in the ratio of tryptophan to large neutral amino acids in the blood. This reduces competition with other amino acids for the Large Neutral Amino Acid Transporter protein for uptake of tryptophan across the blood-brain barrier into the central nervous system. Once inside the central nervous system, tryptophan is converted into serotonin by the raphe nuclei, and serotonin is further metabolised into melatonin by the pineal gland.
It is found in turkey at a level typical of poultry in general [1].
Alcoholic beverage consumption at holiday feasts is likely to compound the effect.
Medicinal uses
5-Hydroxytryptophan (5-HTP), a metabolite of tryptophan, has been suggested as a treatment for epilepsy[11] and depression though clinical trials are inconclusive and lacking.[12]
5-HTP readily crosses the blood brain barrier and in addition is rapidly decarboxylated to serotonin (5-hydroxytryptamine or 5-HT)[13] and therefore may useful for the treatment of depression. However serotonin has a relatively short half life since it is rapidly metabolized by monoamine oxidase therefore is likely to have limited efficacy. It is marketed in Europe for depression and other indications under brand names like Cincofarm and Tript-OH.
In the United States, 5-HTP does not require a prescription as it is covered under the Dietary Supplement Act. However, since the quality of dietary supplements are not regulated by the FDA, the quality of dietary and nutritional supplements tends to vary and there is no guarantee that the label accurately depicts what the bottle contains.[citation needed]
In recent years, compounding pharmacies and some mail-order supplement retailers have begun selling tryptophan to the general public. Tryptophan has also remained on the market as a prescription drug (Tryptan) which some psychiatrists continue to prescribe, particularly as an augmenting agent for people who are unresponsive to antidepressant drugs.[citation needed] Also, most health-food stores sell 5-HTP to get around the resulting artificially high cost of the amino acid itself. But several high quality sources of L-Tryptophan do exist, and are sold in many of the largest health food stores nationwide. Indeed, tryptophan has continued to be used in clinical and experimental studies employing human patients and subjects. Several of these studies suggest tryptophan can effectively treat the fall/winter depression variant of seasonal affective disorder.[14]
Fictional references
- In James Cameron's TV series Dark Angel, genetically engineered Max Guevara and the other escaped X-5s need to take tryptophan supplements to control their seizures which were the result of a faulty gene.
- In an episode of Seinfeld, Jerry and George use turkey and boxed wine to cause Jerry's girlfriend to fall asleep so they can play with her extensive antique toy collection. When Jerry's girlfriend asks what is it in turkey that makes people drowsy, Jerry and George immediately and simultaneously respond "Tryptophan!"
- In the TV series Reno 911!, the faux-information documentary "Keeping it Real, Real Safe" claims that tryptophan is as dangerous as alcohol when it comes to driving.
- In the episode "Psycho Therapy" of the MTV animated series Daria, Daria tells her father, Jake, of tryptophan in milk and its calming influences. This serves as a running gag through the episode.
- In the TV series "Titus", Christopher Titus believed it was tryptophan that caused sleepiness during a turkey dinner. It was in fact the bottle of antidepressants his mother put in their food.
ReferencesISBN links support NWE through referral fees
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedFolling - ↑ Wurtman RJ, Shoemaker WJ, Larin F (1968). Mechanism of the daily rhythm in hepatic tyrosine transaminase activity: role of dietary tryptophan. Proc Natl Acad Sci U S A 59 (3): 800-7.
- ↑ Smith, Jeffrey M (September 2003). "Chapter 4: Deadly Epidemic", Seeds of Deception: Exposing Industry and Government Lies About the Safety of the Genetically Engineered Foods You're Eating. Fairfield, Iowa 52556: Yes! Books, pages 107-127. ISBN 978-0972966580.
- ↑ Mayeno AN, Gleich GJ (1994). Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale. Trends Biotechnol 12 (9): 346-52.
- ↑ James Maryanski. FDA. July 5, 1996.
- ↑ Page S. Center for Food Safely and Applied Nutrition, FDA, Congressional Hearing, Subcommittee, July 18, 1991.
- ↑ Beisler JH (2000). Dietary Supplements and Their Discontents: FDA Regulation and the Dietary Supplement Health and Education Act of 1994 (L-tryptophan Section). Rutgers Law Journal.
- ↑ FDA Tryptophan Recall
- ↑ Raphals P (2000). Does medical mystery threaten biotech?. Science 250: 4981.
- ↑ FDA Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan
- ↑ Kostowski W, Bidzinski A, Hauptmann M, Malinowski JE, Jerlicz M, Dymecki J (1978). Brain serotonin and epileptic seizures in mice: a pharmacological and biochemical study. Pol J Pharmacol Pharm 30 (1): 41-7.
- ↑ Turner EH, Loftis JM, Blackwell AD (2006). Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther 109 (3): 325-38.
- ↑ Hardebo JE, Owman C (1980). Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann NeurolAnn Neurol 8 (1): 1-31.
- ↑ Jepson TL, Ernst ME, Kelly MW (1999). Current perspectives on the management of seasonal affective disorder. J Am Pharm Assoc (Wash) 39 (6): 822-9.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
[1]).
See also
- 5-hydroxytryptophan
- Tryptamines
External links
- Tryptophan catabolism (early stages)
- Tryptophan catabolism (later stages)
- Computational Chemistry Wiki
- Thanksgiving, Turkey, and Tryptophan
- FDA Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan
- Snopes article debunking the turkey–drowsiness connection
- The FDA Ban of L-Tryptophan: Politics, Profits and Prozac
- Effects of Tryptophan Depletion on the Performance of an Iterated Prisoner's Dilemma Game in Healthy Adults - Nature Neuropsychopharmacology
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Template:Tryptamines
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Pallaghy PK, Melnikova AP, Jimenez EC, Olivera BM, Norton RS (1999). Solution structure of contryphan-R, a naturally occurring disulfide-bridged octapeptide containing D-tryptophan: comparison with protein loops. Biochemistry 38 (35): 11553-9.
- ↑ David W. Brooks 2007 Teaching and Research Web Site Intrinsic Fluorescence of Proteins and Peptides
- ↑ Vivian JT, Callis PR (2006). Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J 80 (5): 2093-109.