Protein
Alongside polysaccharides, lipids, and nucleic acids, proteins are a major class of the bio-macromolecules that make up the primary constituents of biological organisms. A protein is a complex, high-molecular-mass, organic compound that consists of amino acids joined by peptide bonds. As suggested by the Greek origins of the term (from the word protas meaning "of primary importance"), proteins are essential to the structure and function of all living cells and viruses.
Different proteins perform a wide variety of biological functions:
- Some proteins are enzymes, which catalyze chemical reactions.
- Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton, which is like a system of scaffolding within a cell.
- Still more functions filled by proteins include immune response
- the storage and transport of various ligands.
Proteins are essentially polymers made up of a specific sequence of amino acids. The details of this sequence are stored in the code of a gene. Through the processes of transcription and translation, a cell reads the genetic information and uses it to construct the protein. In many cases, the resulting protein is then chemically altered (post-translational modification), before becoming functional. It is very common for proteins to work together to achieve a particular function, and often physically associate with one another to form a complex.
In nutrition, proteins are broken down through digestion back into free amino acids for the organism, including those the organism may not be able to synthesize itself. explain need for dietary protein to get essential amino acids
Proteins are among the most actively-studied molecules in biochemistry, and were discovered by Jöns Jakob Berzelius in 1838. expand to include some reasons for study of proteins and major areas of investigation.
The structure of proteins
Components and synthesis
Proteins are polymers built from 20 different L-alpha-amino acids. (more on amino acids).
Amino acid sequence is the link between the genetic code and the three-dimensional structure of proteins that determines their function. Proteins are assembled from amino acids using information present in genes. Genes are transcribed into RNA, RNA is then subject to post-transcriptional modification and control, resulting in a mature mRNA that undergoes translation into a protein.
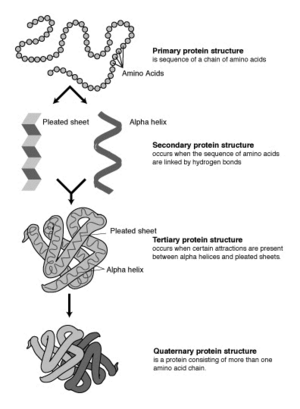
The four levels of protein structure
Proteins fold into unique three-dimensional structures. The shape into which a protein naturally folds is known as its native state, which is determined by its sequence of amino acids. Sometimes, however, proteins do not fold properly. When proteins misfold, it can lead to illnesses such as Alzheimer’s disease, in which brain function is limited by deposits of proteins that have misfolded and can no longer perform their functions. A full understanding of how proteins fold (and why protein misfolding occurs) might lead to advances in the treatment of diseases like Alzheimer’s.
Biochemists refer to four distinct aspects of a protein's structure:
- Primary structure is the linear amino acid sequence encoded by DNA. Any error in this sequence, such as the substitution of one amino acid residue for another, may lead to a congenital disease.
- Secondary structures are highly patterned sub-structures that form in the interaction of amino acid residues near to each other on the chain. The most common are the alpha helix and the beta sheet. Secondary structures are defined, meaning that there can be many different secondary motifs present in one single protein molecule.
- Tertiary structure refers to the overall, three-dimensional shape of a single protein molecule. This spatial relationship of amino acid residues that are far apart on the sequence is primarily formed by hydrophobic interactions, though hydrogen bonds, ionic interactions, and disulfide bonds are usually involved as well.
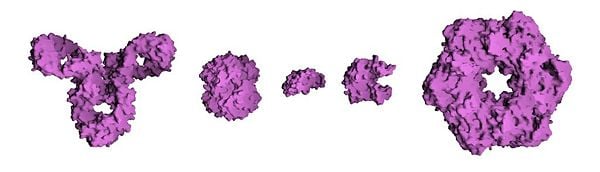
- Some proteins may have a quaternary structure, i.e., the shape or structure that results from the union of more than one protein molecule (called ‘’subunits’’ in this context) which function as part of the larger assembly, or protein complex. Hemoglobin, which serves as an oxygen carrier in blood, has a quaternary structure of four subunits.
In addition to these levels of structure, proteins may shift between several similar structures in performing their biological function. In the context of these functional rearrangements, tertiary or quaternary structures are usually referred to as "conformations," and transitions between them are called conformational changes. Although any unique polypeptide may have more than one stable folded conformation, each conformation has its own biological activity and only one conformation is considered to be the active one. This assumption has been recently challenged by the discovery of intrinsically unstructured proteins, which can fold in multiple structures with different biological activity.
Diversity
Proteins are generally large molecules, having molecular masses of up to 3,000,000 (the muscle protein titin has a single amino acid chain 27,000 subunits long) however protein masses are generally measured in kiloDaltons (kDa). Such long chains of amino acids are almost universally referred to as proteins, but shorter strings of amino acids are referred to as "polypeptides," "peptides" or rarely, "oligopeptides". The dividing line is undefined, though "polypeptide" usually refers to an amino acid chain lacking tertiary structure which may be more likely to act as a hormone (like insulin), rather than as an enzyme (which depends on its defined tertiary structure for functionality).
Major functions of proteins
Proteins are involved in practically every function performed by a cell, including regulation of cellular functions such as signal transduction and metabolism. However, several major classes of proteins may be identified based on the functions below:
- Enzyme catalysis. Nearly all of the chemical reactions in living organisms—from the initial breakdown of food nutrients in the saliva by X and Y, to the replication of an entire chromosome (true?)—are catalyzed by proteins.
- Transport and storage. Membrane-associated proteins move their substrates (such as small molecules and ions) from place to place without altering their chemical properties. E.g., the protein hemoglobin (pictured above) transports oxygen in blood.
- Immune protection. Antibodies, the basis of the adaptive immune system, are soluble proteins capable of recognizing and combining with foreign substances. This class also includes toxins, which play a defensive role (e.g., the dendrotoxins of snakes).
- Signaling. Receptors mediate the responses of nerve cells to specific stimuli. Rhodopsin, for example, is a light sensitive protein in the rod cells of the retina of vertebrates.
- Structural support. Examples include tubulin, actin, collagen and keratin, which are important strengthening components of skin, hair, and bone.
- Coordinated motion. Another special class of proteins consists of motor proteins such as myosin, kinesin, and dynein. These proteins are "molecular motors," generating physical force which can move organelles, cells, and entire muscles. Proteins are the major components of muscle, and muscle contraction involves the sliding motion of two kinds of protein filaments. At the microscopic level, the propulsion of sperm by flagella is produced by protein assemblies.
- Control of growth and differentiation. In higher organisms, growth factor proteins control the growth and differentiation of cells (e.g., insulin). regulatory proteins, such as transcription factors or cyclins that regulate the cell cycle?
Proteins in the human diet
Protein is an important macronutrient to the human diet, supplying the body's needs for amino acids, the building blocks of proteins. Mammals cannot synthesize all 20 standard amino acids, so protein from the diet is necessary to acquire those that cannot be synthesized, known as essential amino acids. Between 8 and 10 amino acids are considered essential in humans.
While animal meats are rich sources of this vital dietary element, protein is also found in plant foods, such as grains and legumes, and in eggs and dairy products, such as milk and yogurt. The best way to obtain the full range of essential amino acids is to consume a variety of protein-rich foods. Soy products such as tofu are particularly important to many vegetarians and vegans as a source of complete protein (a protein that contains significant amounts of all the essential amino acids).
The exact amount of dietary protein needed to satisfy protein requirements for humans, known as an RDA, may vary widely depending on age, sex, level of physical activity, and medical condition.
Protein deficiency
Protein deficiency can lead to symptoms such as fatigue, insulin resistance, hair loss, loss of hair pigment, loss of muscle mass, low body temperature, hormonal irregularities, as well as loss of skin elasticity. Severe protein deficiency, encountered only in times of famine, is fatal, due to the lack of material for the body to construct its own proteins (see kwashiorkor).
Protein deficiency is rare in developed countries, but it can occur in people who are dieting to lose weight, or in older adults, who may have a poor diet. Convalescent people recovering from surgery, trauma, or illness may become protein deficient if they do not increase their intake to support their increased needs. A deficiency can also occur if the protein you eat is incomplete and fails to supply all the essential amino acids.
Example of migratory bird (….)
Other dietary imbalances
Some suspect excessive protein intake is linked to several problems:
- Overreaction within the immune system
- Liver dysfunction due to increased toxic residues. Because the body is unable to store excess protein, it is broken down and converted into sugars or fatty acids. The liver removes nitrogen from the amino acids, so that they can be burned as fuel, and the nitrogen is incorporated into urea, the substance that is excreted by the kidneys. These organs can normally cope with any extra workload but if kidney disease occurs, a decrease in protein will often be prescribed.
- Loss of bone density, frailty of bones is due to calcium and glutamine being leached from bone and muscle tissue to balance increased acid intake from diet (blood pH is maintained at around 7.4). This effect is not present if intake of alkaline minerals (from fruits and vegetables, cereals are acidic as are proteins, fats are neutral) is high. In such cases, protein intake is anabolic to bone.
Proteins are often progenitors in allergies and allergic reactions to certain foods. This is because the structure of each form of protein is slightly different; some may trigger a response from the immune system while others remain perfectly safe. Many people are allergic to casein, the protein in milk; gluten, the protein in wheat and other grains; the particular proteins found in peanuts; or those in shellfish or other seafoods. It is extremely unusual for the same person to adversely react to more than two different types of proteins, due to the diversity between protein or amino acid types.
Studying proteins
The first mention of the word protein was from a letter sent by Jöns Jakob Berzelius to Gerhardus Johannes Mulder on 10. July 1838, where he wrote:
- «Le nom protéine que je vous propose pour l’oxyde organique de la fibrine et de l’albumine, je voulais le dériver de πρωτειος, parce qu’il paraît être la substance primitive ou principale de la nutrition animale.»
Translated as:
- "The name protein that I propose for the organic oxide of fibrin and albumin, I wanted to derive from [the Greek word] πρωτειος, because it appears to be the primitive or principal substance of animal nutrition."
Investigation of proteins and their properties had been going on since about 1800 when scientists were finding the first signs of this, at the time, unknown class of organic compounds.
Proteins are sensitive to their environment. They may only be active in their native state, over a small pH range, and under solution conditions with a minimum quantity of electrolytes. A protein in its native state is often described as folded. A protein that is not in its native state is said to be denatured. Denatured proteins generally have no well-defined secondary structure. Many proteins denature and will not remain in solution in distilled water.
One of the more striking discoveries of the 20th century was that the native and denatured states in many proteins were interconvertible, that by careful control of solution conditions (by for example, dialyzing away a denaturing chemical), a denatured protein could be converted to native form. The issue of how proteins arrive at their native state is an important area of biochemical study, called the study of protein folding.
Through genetic engineering, researchers can alter the sequence and hence the structure, "targeting", susceptibility to regulation and other properties of a protein. The genetic sequences of different proteins may be spliced together to create "chimeric" proteins that possess properties of both. This form of tinkering represents one of the chief tools of cell and molecular biologists to change and to probe the workings of cells. Another area of protein research attempts to engineer proteins with entirely new properties or functions, a field known as protein engineering.
The most commonly used test for the amount of proteins in foods is called the Kjeldah test. This test determines the total nitrogen in a sample. The only major component of most food which contains nitrogen is protein (fat, carbohydrate and dietary fibre do not contain nitrogen). If the amount of nitrogen is multiplied by a factor depending on the kinds of protein expected in the food the total protein can be determined. On food labels the protein is given by the nitrogen muliplied by 6.25, because the average nitrogen content of proteins is about 16%. The reason the Kjeldah test is used is because it is the method the AOAC International has adopted and which is therefore used by many food standards agencies around the world.
ReferencesISBN links support NWE through referral fees
- Atkins, Peter and Loretta Jones. 2005. Chemical Principles, 3rd edition. New York, NY: W.H. Freeman.
- Stryer, Lubert. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
- The Protein Databank
- UniProt the Universal Protein Resource
- Human Protein Atlas
- iHOP - Information Hyperlinked over Proteins
- Proteins: Biogenesis to Degradation - The Virtual Library of Biochemistry and Cell Biology
- MIT's Laboratory for Protein Molecular Self-Assembly
- Numerous publications on synthetic biomimetic protein-based biomaterials
- Amino acid metabolism
- Protein Images
- Online Protein viewer with a local PDB database
- NCBI Entrez Protein database
- NCBI Protein Structure database
- TheDailyPlate.com - Protein content details for over 100,000 foods.
- AOAC International
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.