Ligand
In chemistry, a ligand is an atom, ion, or molecule that generally donates one or more of its electrons through a coordinate covalent bond to, or shares its electrons through a covalent bond with, one or more central atoms or ions. These ligands act as Lewis bases. In a smaller number of cases, a ligand is a molecule that accepts electrons from a Lewis base. In other words, the ligand acts as a Lewis acid.
Most commonly, the central atom is a metal or metalloid in inorganic chemistry. But in organic chemistry, ligands are also used to protect functional groups or to stabilize reactive compounds. For instance, borane (BH3) is a ligand for the protection of phosphine (PH3). Tetrahydrofuran (THF) can be used as a ligand for BH3, to make BH3 more stable and easier to handle. The molecule resulting from the coordination of a ligand (or an array of ligands) to a central atom is called a complex. The ligands in a complex stabilize the central atom, and dictate the reactivity of the central atom. Factors that characterize the ligands are their charge, size (bulk), and the nature of the constituent atoms.
Ligands in metal complexes
The constitution of metal complexes has been described by Alfred Werner, who developed the basis for modern coordination chemistry. Ligands that are directly bonded to the metal (that is, share electrons) are called "inner sphere" ligands. If the inner-sphere ligands do not balance the charge of the central atom (the oxidation number), this may be done by simple ionic bonding with another set of counter ions (the "outer-sphere" ligands). The complex of the metal with the inner sphere ligands is then called a complex ion (which can be either cationic or anionic). The complex, along with its counter ions, is called a coordination compound. The size of a ligand is indicated by its cone angle.
Donation and back-donation
In general, ligands donate electron density to the (electron deficient) central atom—that is, they overlap between the highest occupied molecular orbital (HOMO) of the ligand with the lowest unoccupied molecular orbital (LUMO) of the central atom. The ligand thus acts as a Lewis base by donating electron density (in general, electron pairs) to the central atom, acting as a Lewis acid. In some cases, ligands donate only one electron from a singly occupied orbital (the donating atom in these ligands is a radical).
Some metal centers in combination with certain ligands (e.g. carbon monoxide (CO)) can be further stabilized by donating electron density back to the ligand in a process known as back-bonding. In this case, a filled, central-atom-based orbital donates density into the LUMO of the (coordinated) ligand.
Strong field and weak field ligands
Ligands and metal ions can be ordered by their 'hardness' (see also hard soft acid base theory). Certain metal ions have a preference for certain ligands. In general, 'hard' metal ions prefer weak field ligands, whereas 'soft' metal ions prefer strong field ligands. From a molecular orbital theory point of view, the HOMO of the ligand should have an energy that makes overlap with the LUMO of the metal preferential. Metal ions bound to strong-field ligands follow the Aufbau principle, whereas complexes bound to weak-field ligands follow Hund's rule.
Binding of the metal with the ligands results in a set of molecular orbitals, where the metal can be identified with a new HOMO and LUMO (the orbitals defining the properties and reactivity of the resulting complex) and a certain ordering of the five d-orbitals (which may be filled, or partially filled with electrons). In an octahedral environment, the five otherwise degenerate d-orbitals split in sets of two and three orbitals.
- three orbitals of low energy: dxy, dxz and dyz
- two of high energy: dz2 and dx2-y2
The energy difference between these two sets of d-orbitals is called the splitting parameter, Δo. The magnitude of Δo is determined by the field-strength of the ligand: strong field ligands, by definition, increase Δo more than weak field ligands. Ligands can now be sorted according to the magnitude of Δo (see the table below). This ordering of ligands is almost invariable for all metal ions and is called spectrochemical series.
For complexes with a tetrahedral surrounding, the d-orbitals again split into two sets, but this time in reverse order:
- two orbitals of low energy: dz2 and dx2-y2
- three orbitals of high energy: dxy, dxz and dyz
The energy difference between these two sets of d-orbitals is now called Δt. The magnitude of Δt is smaller than for Δo, because in a tetrahedral complex only four ligands influence the d-orbitals, whereas in an octahedral complex the d-orbitals are influenced by six ligands. When the coordination number is neither octahedral nor tetrahedral, the splitting becomes correspondingly more complex. For the purposes of ranking ligands, however, the properties of the octahedral complexes and the resulting Δo has been of primary interest.
The arrangement of the d-orbitals on the central atom (as determined by the 'strength' of the ligand), has a strong effect on virtually all the properties of the resulting complexes—the energy differences in the d-orbitals has a strong effect in the optical absorption spectra of metal complexes. It turns out that valence electrons occupying orbitals with significant three d-orbital character absorb in the 400-800 nm region of the spectrum (UV-visible range). The absorption of light (what we perceive as the color) by these electrons (that is, excitation of electrons from one orbital to another orbital under influence of light) can be correlated to the ground state of the metal complex, which reflects the bonding properties of the ligands. The relative change in (relative) energy of the d-orbitals as a function of the field-strength of the ligands is described in Tanabe-Sugano diagrams.
Denticity
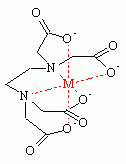
Some ligand molecules are able to bind to the metal ion through multiple sites, often because they have free lone pairs on more than one atom. Ligands that bind to more than one site are termed chelating (from the Greek for claw). For example, a ligand binding through two sites is bidentate and three sites is tridentate. The bite angle refers to the angle between the two bonds of a bidentate chelate. Chelating ligands are commonly formed by linking donor groups via organic linkers. A classic example is ethylene diamine, which is derived by the linking of two ammonia groups with an ethylene (-CH2CH2-) linker. A classic example of a polydentate ligand is the hexadentate chelating agent EDTA. It is able to bond through six sites, completely surrounding some metals. The number of atoms with which a polydentate ligand bind to the metal center is called its denticity (symbol κ). κ indicates the number non-contiguous donor sites by which a ligand attaches to a metal. In catalysis the effectiveness of a chelating system depends on the chelating angle or bite angle.
Hapticity vs denticity
Hapticity (η) and denticity are often confused. Hapticity refers to contiguous atoms that are attached to a metal. Ethylene forms η2 complexes because two adjacent carbon atoms bind to the metal. Ethylenediamine forms κ2 complexes. Cyclopentadienyl is typically bonded in η5 mode because all five carbon atoms are bonded to the metal. EDTA4- on the other hand, when it is sexidentate, is κ6 mode, the amines and the carboxylate oxygen atoms are not connected directly. To simplify matters, ηn tends to refer to unsaturated hydrocarbons and κn tends to describe polydentate amine and carboxylate ligands.
Complexes of polydentate ligands are called chelate complexes. They tend to be more stable than complexes derived from monodentate ligands. This enhanced stability is attributed to the necessity to break all of the bonds to the central atom for the hexadentate ligand to be displaced. This increased stability or inertness is called the chelate effect. In terms of the enhanced thermodynamic stability of chelate complexes, entropy favors the displacement of many ligands by one polydentate ligand. The increase in the total number of molecules in solution is favorable.
Related to the chelate effect is the macrocyclic effect. A macrocyclic ligand is any large cyclic ligand which at least partially surrounds the central atom and bonds to it, leaving the central atom at the centre of a large ring. The more rigid and the higher its denticity, the more inert will be the macrocyclic complex. Heme is a good example, the iron atom is at the centre of a porphyrin macrocycle, being bound to four nitrogen atoms of the tetrapyrrole macrocycle. The very stable dimethylglyoximate complex of nickel is a synthetic macrocycle derived from the anion of dimethylglyoxime.
Unlike polydentate ligands, ambidentate ligands can attach to the central atom in two places but not both. A good example of this is thiocyanide, SCN-, which can attach at either the sulfur atom or the nitrogen atom. Such compounds give rise to linkage isomerism.
Common ligands
- See Complex (chemistry).
Virtually every molecule and every ion can serve as a ligand for (or "coordinate to") metals. Monodentate ligands include virtually all anions and all simple Lewis bases. Thus, the halides and pseudohalides are important anionic ligands whereas ammonia, carbon monoxide, and water are particularly common charge-neutral ligands. Simple organic species are also very common, be they anionic (RO- and RCO2-) or neutral (R2O, R2S, R3-xNHx, and R3P). The steric properties of some ligands are evaluated in terms of their cone angles.
Beyond the classical Lewis bases and anions, all unsaturated molecules are also ligands, utilizing their π-electrons in forming the coordinate bond. Also, metals can bind to the σ bonds in for example silanes, hydrocarbons, and dihydrogen (see also: agostic interaction).
In complexes of non-innocent ligands, the ligand is bonded to metals via conventional bonds, but the ligand is also redox-active.
Examples of common ligands (by field strength)
In the following table, ligands are sorted by field strength (weak field ligands first):
| Ligand | formula (bonding atom(s) in bold) | Charge | Most common denticity | Remark(s) |
|---|---|---|---|---|
| Iodide | I- | monoanionic | monodentate | |
| Bromide | Br- | monoanionic | monodentate | |
| Sulphide | S2- | dianionic | monodentate (M=S), or bidentate bridging (M-S-M') | |
| Thiocyanate | S-CN- | monoanionic | monodentate | ambidentate (see also isothiocyanate, vide infra) |
| Chloride | Cl- | monoanionic | monodentate | also found bridging |
| Nitrate | O-NO2- | monoanionic | monodentate | |
| Azide | N-N2- | monoanionic | monodentate | |
| Fluoride | F- | monoanionic | monodentate | |
| Hydroxide | O-H- | monoanionic | monodentate | often found as a bridging ligand |
| Oxalate | [O-C(=O)-C(=O)-O]2- | dianionic | bidentate | |
| Water | H-O-H | neutral | monodentate | monodentate |
| Isothiocyanate | N=C=S- | monoanionic | monodentate | ambidentate (see also thiocyanate, vide supra) |
| Acetonitrile | CH3CN | neutral | monodentate | |
| Pyridine | C5H5N | neutral | monodentate | |
| Ammonia | NH3 | neutral | monodentate | |
| Ethylenediamine | en | neutral | bidentate | |
| 2,2'-Bipyridine | bipy | neutral | bidentate | easily reduced to its (radical) anion or even to its dianion |
| 1,10-Phenanthroline | phen | neutral | bidentate | |
| Nitrite | O-N-O- | monoanionic | monodentate | ambidentate |
| Triphenylphosphine | PPh3 | neutral | monodentate | |
| Cyanide | CN- | monoanionic | monodentate | can bridge between metals (both metals bound to C, or one to C and one to N) |
| Carbon monoxide | CO | neutral | monodentate | can bridge between metals (both metals bound to C) |
Note: The entries in the table are sorted by field strength, binding through the stated atom (i.e. as a terminal ligand), the 'strength' of the ligand changes when the ligand binds in an alternative binding mode (e.g. when it bridges between metals) or when the conformation of the ligand gets distorted (e.g. a linear ligand that is forced through steric interactions to bind in a non-linear fashion).
Other generally encountered ligands (in alphabetical order)
In this table, other common ligands are listed in alphabetical order.
| Ligand | formula (bonding atom(s) in bold) | Charge | Most common denticity | Remark(s) |
|---|---|---|---|---|
| Acetylacetonate (Acac) | CH3-C(O)-CH-C(O)-CH3 | monoanionic | bidentate | In general bidentate, bound through both oxygens, but sometimes bound through the central carbon only, see also analogous ketimine analogues |
| Alkenes | R2C=CR2 | neutral | compounds with a C-C double bond | |
| Benzene | C6H6 | neutral | and other arenes | |
| 1,2-Bis(diphenylphosphino)ethane (dppe) | Ph2PC2H4PPh2 | neutral | bidentate | |
| Corroles | tetradentate | |||
| Crown ethers | neutral | primarily for alkali and alkaline earth metal cations | ||
| 2,2,2-crypt | hexadentate | primarily for alkali and alkaline earth metal cations | ||
| Cryptates | neutral | |||
| Cyclopentadienyl | [C5H5]- | monoanionic | ||
| Diethylenetriamine (dien) | neutral | tridentate | related to TACN, but not constrained to facial complexation | |
| Dimethylglyoximate (dmgH-) | monoanionic | |||
| Ethylenediaminetetraacetate (EDTA) | tetra-anionic | hexadentate | actual ligand is the tetra-anion | |
| Ethylenediaminetriacetate | trianionic | pentadentate | actual ligand is the trianion | |
| glycinate | bidentate | other α-amino acid anions are comparable (but chiral) | ||
| Heme | dianionic | tetradentate | macrocyclic ligand | |
| Nitrosyl | NO+ | cationic | bent (1e) and linear (3e) bonding mode | |
| Scorpionate ligand | tridentate | |||
| Sulfite | monoanionic | monodentate | ambidentate | |
| 2,2',5',2-Terpyridine (terpy) | neutral | tridentate | meridional bonding only | |
| Thiocyanate | monoanionic | monodentate | ambidentate, sometimes bridging | |
| Triazacyclononane (tacn) | (C2H4)3(NR)3 | neutral | tridentate | macrocyclic ligand see also the N,N',N"-trimethylated analogue |
| Triethylenetetramine (trien) | neutral | tetradentate | ||
| Tris(2-aminoethyl)amine (tren) | neutral | tetradentate | ||
| Tris(2-diphenylphosphineethyl)amine (np3) | neutral | tetradentate | ||
| Terpyridine | neutral | tridentate |
See also
- Chelation
- Complex (chemistry)
- Functional group
- Inorganic chemistry
- Organic chemistry
- Organometallic chemistry
ReferencesISBN links support NWE through referral fees
- Astruc, Didier. 2007. Organometallic Chemistry and Catalysis. Berlin: Springer.
- Crabtree, Robert H. 2005. The Organometallic Chemistry of the Transition Metals, 4th ed. Hoboken, NJ: Wiley. ISBN 978-0471662563
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052
- Solomons, T.W. Graham, and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998
- Zumdahl, Steven S. 2005. Chemical Principles. New York, NY: Houghton Mifflin. ISBN 0618372067
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.