Olivine
| Olivine | |
|---|---|
 |
|
| General | |
| Category | Mineral |
| Chemical formula | (Mg, Fe)2SiO4 |
| Identification | |
| Color | Yellow to yellow-green |
| Crystal system | Orthorhombic |
| Cleavage | Poor |
| Fracture | Conchoidal |
| Mohs Scale hardness | 6.5-7 |
| Luster | Vitreous |
| Refractive index | 1.64-1.70 |
| Birefringence | +0.036 |
| Streak | White |
| Specific gravity | 3.2-4.3 |
| {{{density}}} | |
The name olivine (or chrysolite or evening emerald) is given to a series of minerals that are among the most common on Earth. The gem-quality variety is known as peridot. These minerals have also been identified on the Moon, Mars, and comet Wild 2.
Chemically, members of this series are composed of magnesium iron silicate ((Mg,Fe)2SiO4). The ratio of magnesium to iron varies between the two endmembers of the series: forsterite (Fo) (magnesium silicate) and fayalite (Fa) (iron silicate).
Members of this series usually range in color from a nearly emerald green to a pale yellowish green. They have a vitreous (glassy) luster and vary from transparent to translucent. In addition, some specimens are nearly colorless and are likely to approach forsterite in composition; others are greenish brown to black and approach pure fayalite in composition.
The name olivine is also used for a group of minerals with related structures. They include tephroite (manganese silicate, Mn2SiO4), monticellite (calcium magnesium silicate, CaMgSiO4), and kirschsteinite (calcium iron silicate, CaFeSiO4).
Etymology
Olivine was named for the olive-green color of many of the specimens. The word peridot comes from the French word for olivine, and chrysolite, from the Greek words for gold and stone.
Formation and occurrence
Olivine occurs in both mafic and ultramafic igneous rocks and as a primary mineral in certain metamorphic rocks. Magnesium-rich olivine crystallizes from magma that is rich in magnesium and low in silica. That magma crystallizes to mafic rocks such as gabbro and basalt. Ultramafic rocks such as peridotite, and dunite can be residues left after extraction of magmas, and typically they are more enriched in olivine after extraction of partial melts. Olivine, or high pressure structural variants, constitute over 50% of the Earth's upper mantle, making it one of the Earth's most common minerals by volume. The metamorphism of impure dolomite or other sedimentary rocks with high magnesium and low silica content also produces magnesium-rich olivine, or forsterite.
Iron-rich olivine is relatively much less common, but it occurs in igneous rocks in small amounts in rare granites and rhyolites, and extremely iron-rich olivine can exist stably with quartz and tridymite. By contrast, magnesium-rich olivine does not occur stably with silica minerals, as it would react with them to form orthopyroxene ((Mg,Fe)2Si2O6).
Magnesium-rich olivine has also been discovered in meteorites, on Mars, and on Earth's Moon. Such meteorites include chondrites (collections of debris from the early Solar System), and pallasites (mixes of iron-nickel and olivine). The spectral signature of olivine has been seen in the dust disks around young stars. The tails of comets (which formed from the dust disk around the young Sun) often have the spectral signature of olivine, and the presence of olivine has recently been verified in samples of a comet from the Stardust spacecraft.[1]
Some of the finest gem-quality olivine has been obtained from a body of mantle rocks on Zabargad island in the Red Sea.
Characteristics
The green color of olivine is thought to be a result of traces of nickel). It may alter to a reddish color from the oxidation of iron. The mineral has a conchoidal fracture and is rather brittle. Its hardness is 6.5–7 on the Mohs scale, and its relative density is 3.27–3.37.
The compositions of members of the series are commonly expressed as molar percentages of forsterite and fayalite. For instance, Fo70Fa30 represents a 70:30 molar ratio, in a combination of forsterite and fayalite.
Forsterite has an unusually high melting temperature: almost 1900 °C at atmospheric pressure. The melting temperature of fayalite is much lower, about 1200 °C. The melting temperature varies smoothly between the two endmembers, as do other properties.
Crystal structure
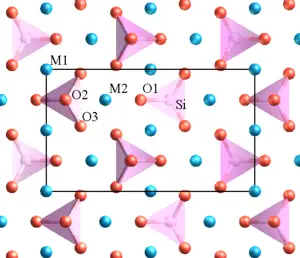
Minerals in the olivine group crystallize in the orthorhombic system (space group Pbnm) with isolated silicate tetrahedra. From this view, olivine is classified as a nesosilicate. In an alternative view, the atomic structure can be described as a hexagonal, close-packed array of oxide ions, with half the octahedral sites occupied by magnesium or iron ions and one-eighth of the tetrahedral sites occupied by silicon ions.
There are three distinct oxygen sites (marked O1, O2, and O3 in figure 1), two distinct metal sites (M1 and M2), and only one distinct silicon site. O1, O2, M2, and Si all lie on mirror planes, while M1 exists on an inversion center. O3 lies in a general position.
High pressure polymorphs and phase diagram
At the high temperatures and pressures found at depth within the Earth, the structure of olivine is no longer stable. Below depths of about 410 kilometers (km), olivine undergoes a phase transition to the sorosilicate, wadsleyite, and, at about 520-km depth, wadsleyite transforms into ringwoodite, which has the spinel structure. These phase transitions lead to a discontinuous increase in the density of the Earth's mantle that can be observed by seismic methods.
The pressure at which these phase transitions occur depends on temperature and iron content (Deer et al., 1992). At 800°C, the pure magnesium endmember, forsterite, transforms to wadsleyite at 11.8 gigapascals (118 kbar) and to ringwoodite at pressures above 14 GPa (140 kbar). Increasing the iron content decreases the pressure of the phase transition and narrows the wadsleyite stability field. At about 0.8 mole fraction fayalite, olivine transforms directly to ringwoodite over the pressure range 10–11.5 GPa (100–115 kbar). Fayalite transforms to Fe2SiO4 spinel at pressures below 5 GPa (50 kbar). Increasing the temperature increases the pressure of these phase transitions.
Historical and cultural significance
- According to Rebbenu Bachya, the word "tarshish" in the verse Exodus 28:20 means "chrysolite" and was the stone on the Ephod representing the tribe of Asher.
- Peridot is the birthstone of August.
See also
Notes
- ↑ NASA Press Release. 2006. NASA's Stardust Findings May Alter View of Comet Formation. NASA Jet Propulsion Laboratory, California Institute of Technology. Retrieved April 9, 2007.
ReferencesISBN links support NWE through referral fees
- Deer, W.A., R.A. Howie, and J. Zussman. 1996. An Introduction to the Rock-Forming Minerals. 2nd ed. Upper Saddle River, NJ: Prentice Hall. ISBN 0582300940 and ISBN 978-0582300941.
- Farndon, John. 2006. The Practical Encyclopedia of Rocks & Minerals: How to Find, Identify, Collect and Maintain the World's best Specimens, with over 1000 Photographs and Artworks. London: Lorenz Books. ISBN 0754815412 and ISBN 978-0754815419.
- Klein, Cornelis, and Barbara Dutrow. 2007. Manual of Mineral Science. 23rd ed. New York: John Wiley. ISBN 0471721573 and ISBN 978-0471721574.
- Pellant, Chris. 2002. Rocks and Minerals. Smithsonian Handbooks. New York: Dorling Kindersley. ISBN 0789491060 and ISBN 978-0789491060.
- Schumann, Walter. 2006. Gemstones of the World 3rd ed. New York: Sterling. ISBN 1402740166 and ISBN 978-1402740169.
- Shaffer, Paul R., Herbert S. Zim, and Raymond Perlman. 2001. Rocks, Gems and Minerals. Rev. ed. New York: St. Martin's Press. ISBN 1582381321 and ISBN 978-1582381329.
External links
- The Mineral Olivine. Amethyst Galleries. Retrieved April 9, 2007.
- The Olivine Group. Amethyst Galleries. Retrieved April 9, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.