Difference between revisions of "Molecule" - New World Encyclopedia
David Burton (talk | contribs) (Importing newer version) |
Jia Wen Teo (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | In [[chemistry]], a '''molecule''' is an aggregate of at least two atoms in a definite arrangement held together by special forces.<ref>{{cite book|author=Chang, Raymond |title=Chemistry, 6th Ed.|location=New York | publisher=McGraw Hill|year=1998|id=ISBN 0071152210}}</ref> Generally, a molecule is considered the smallest [[list of particles#Molecules|particle]] of a pure [[chemical substance]] that still retains its [[chemical compound|composition]] and chemical properties.<ref> | + | In [[chemistry]], a '''molecule''' is an aggregate of at least two atoms in a definite arrangement held together by special forces.<ref>{{cite book|author=Chang, Raymond |title=Chemistry, 6th Ed.|location=New York | publisher=McGraw Hill|year=1998|id=ISBN |
| + | 0071152210}}</ref> Generally, a molecule is considered the smallest [[list of | ||
| + | particles#Molecules|particle]] of a pure [[chemical substance]] that still retains its | ||
| + | [[chemical compound|composition]] and chemical properties.<ref>http://antoine.frostburg.edu/chem/senese/101/glossary/m.shtml#molecule Molecule Definition]</ref> A molecule may be composed of the same element or atoms of two or more elements joined in a fixed ratio.<ref>{{cite book|author=Chang, Raymond |title=Chemistry, 6th Ed.|location=New York | publisher=McGraw Hill|year=1998|id=ISBN 0071152210}}</ref> In the [[molecular science]]s, a molecule is a sufficiently stable, [[electric charge|electrically]] neutral [[entity]] composed of two or more [[atom]]s.<ref>http://www.iupac.org/goldbook/M04002.pdf IUPAC Defintion of Molecule]</ref> Molecules can be monatomic, diatomic or polyatomic. The concept of "[[monatomic|monatomic molecule]]", i.e. a single-atom as found in [[noble gas]]es, is used almost exclusively in the [[kinetic theory]] of gases.<ref>http://www.ac.wwu.edu/~vawter/PhysicsNet/Topics/Thermal/kinTheoryGas.html] [http://www.usd.edu/phys/courses/phys_111sf/ch_10/10_notes.htm] [http://www.google.com/search?q=monatomic-molecule+kinetic-theory+%2B%22.edu%22]</ref> Exactly two atoms make up diatomic molecules. Examples include O<sub>2</sub>, N<sub>2</sub>, Cl<sub>2</sub>. When diatomic The elements of Group 7A in the periodic table, the [[halogen]]s, exist as diatomic elements. Diatomic molecules of two different elements are polar molecules. This is due to the difference in [[electronegativity]] - the atom with the higher electronegativity pulls the more negatively charged electrons closer to it. The higher concentration of electrons towards one side of the molecule creates a negative charge on that side and a positive charge on the other end. On the other hand, diatomic molecules of like elements have the same electronegativity and are thus nonpolar. | ||
| − | + | Polyatomic molecules contain two or more atoms. H<sub>2</sub>O and NH<sub>3</sub> are some examples. | |
| − | |||
| − | |||
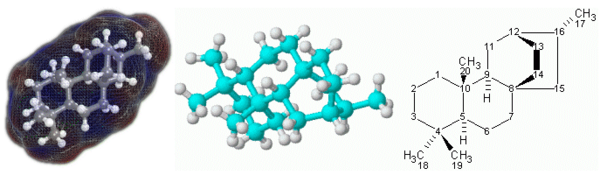
| − | + | [[Image:Atisane3.png|600px|thumb|right|[[3D geometric model|3D]] (left and center) and [[2D | |
| − | |||
| − | + | geometric model|2D]] (right) representations of the [[terpenoid]] molecule [[atisane]].]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Empirical formula == | == Empirical formula == | ||
:''See main article [[empirical formula]]'' | :''See main article [[empirical formula]]'' | ||
| − | The '''empirical formula''' of a molecule is the '''simplest''' [[integer]] [[ratio]] of the [[chemical element]]s that constitute the compound. For example, in their pure forms, [[water]] is always composed of a 2:1 ratio of [[hydrogen]] to [[oxygen]], and ethyl [[alcohol]] or [[ethanol]] is always composed of [[carbon]], [[hydrogen]], and [[oxygen]] in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely - [[dimethyl ether]] has the same ratio as ethanol, for instance. [[Molecules]] with the same [[atom]]s in different arrangements are called [[isomer]]s. The empirical formula is often the same as the molecular formula but not always. For example the molecule [[acetylene]] has molecular formula C<sub>2</sub>H<sub>2</sub>, but the simplest integer ratio of elements is CH. | + | The '''empirical formula''' of a molecule is the '''simplest''' [[integer]] [[ratio]] of the [[chemical element]]s that constitute the compound. For example, in their pure forms, |
| + | [[water]] is always composed of a 2:1 ratio of [[hydrogen]] to [[oxygen]], and ethyl [[alcohol]] or [[ethanol]] is always composed of [[carbon]], [[hydrogen]], and [[oxygen]] in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely - [[dimethyl ether]] has the same ratio as ethanol, for instance. [[Molecules]] with the same [[atom]]s in different arrangements are called [[isomer]]s. The empirical formula is often the same as the molecular formula but not always. For example the molecule [[acetylene]] has molecular formula C<sub>2</sub>H<sub>2</sub>, but the simplest integer ratio of elements is CH. | ||
== Chemical formula == | == Chemical formula == | ||
| Line 26: | Line 22: | ||
The '''chemical formula''' reflects the exact number of atoms that compose a molecule. | The '''chemical formula''' reflects the exact number of atoms that compose a molecule. | ||
| + | |||
| + | == Chemical bond == | ||
| + | :''See main article [[chemical bond]]'' | ||
| + | |||
| + | In a molecule, the atoms are joined by shared pairs of [[electron]]s in a '''chemical bond'''. It may consist of atoms of the same [[chemical element]], as with [[oxygen]] (O<sub>2</sub>), or of different elements, as with [[water (molecule)|water]] (H<sub>2</sub>O). | ||
| + | |||
| + | == Size == | ||
| + | Most molecules are much too small to be seen with the naked eye, but there are exceptions. [[DNA]], a [[macromolecule]], can reach [[macroscopic]] sizes. The smallest molecule is the [[hydrogen]] molecule. The interatomic distance is 0.15 [[nanometre]]s (1.5 [[Angstrom|Å]]). But the size of its [[electron cloud]] is difficult to define precisely. Under [[standard conditions]] molecules have a dimension of a few to several dozen Å. | ||
== Molecular mass == | == Molecular mass == | ||
| Line 31: | Line 35: | ||
The '''[[molecular mass]]''' can be calculated from the chemical formula and is expressed in conventional units equal to 1/12 from the mass of a <sup>12</sup>[[carbon|C]] [[isotope]] atom. For [[network solids]], the term [[formula unit]] is used in [[stoichiometric]] calculations. | The '''[[molecular mass]]''' can be calculated from the chemical formula and is expressed in conventional units equal to 1/12 from the mass of a <sup>12</sup>[[carbon|C]] [[isotope]] atom. For [[network solids]], the term [[formula unit]] is used in [[stoichiometric]] calculations. | ||
| + | |||
| + | == Molecular Models == | ||
== Molecular geometry == | == Molecular geometry == | ||
| Line 36: | Line 42: | ||
Molecules have fixed [[equilibrium]] geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same [[average]] geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its [[reactivity]]. Isomers share a chemical formula but normally have very different properties because of their different structures. [[Stereoisomer]]s, a particular type of isomers, may have very similar physico-chemical properties and at the same time very different [[biochemistry|biochemical]] activities. | Molecules have fixed [[equilibrium]] geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same [[average]] geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its [[reactivity]]. Isomers share a chemical formula but normally have very different properties because of their different structures. [[Stereoisomer]]s, a particular type of isomers, may have very similar physico-chemical properties and at the same time very different [[biochemistry|biochemical]] activities. | ||
| + | |||
| + | == Molecular physics == | ||
| + | The science of molecules is called ''molecular chemistry'' or ''[[molecular physics]]'', depending on the focus. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of [[chemical bond]]s, while molecular physics deals with the laws governing their structure and properties. In practice, | ||
| + | however, this distinction is vague. In molecular sciences, a molecule consists of a stable system ([[bound state]]) comprising two or more [[atom]]s. The term ''unstable molecule'' is | ||
| + | used for very [[reactivity|reactive]] species, i.e., short-lived assemblies ([[resonance]]s) | ||
| + | of [[electron]]s and [[atomic nucleus|nuclei]], such as [[radical (chemistry)|radicals]], | ||
| + | molecular [[ion]]s, [[Rydberg molecule]]s, [[transition state]]s, [[Van der Waals | ||
| + | bonding|Van der Waals complex]]es, or systems of colliding atoms as in [[Bose-Einstein | ||
| + | condensate]]s. A peculiar use of the term ''molecular'' is as a synonym to ''[[covalent bond|covalent]]'', which arises from the fact that, unlike molecular covalent compounds, | ||
| + | [[ionic compound]]s do not yield well-defined ''smallest particles'' that would be | ||
| + | consistent with the definition above. No typical "smallest particle" can be defined for | ||
| + | covalent [[crystal]]s, or [[network solids]], which are composed of repeating [[unit cell]]s that extend indefinitely either in a [[Plane (mathematics)|plane]] (such as in [[graphite]]) three-dimensionally (such as in [[diamond]]). | ||
== Molecular spectroscopy == | == Molecular spectroscopy == | ||
{{main|Spectroscopy}} | {{main|Spectroscopy}} | ||
| − | '''Molecular spectroscopy''' deals with the response ([[frequency spectrum|spectrum]]) of molecules interacting with probing signals of known [[energy]] (or [[frequency]], according to [[Planck's constant|Planck's formula]]). [[Scattering theory]] provides the theoretical background for spectroscopy. | + | '''Molecular spectroscopy''' deals with the response ([[frequency spectrum|spectrum]]) of molecules interacting with probing signals of known [[energy]] (or [[frequency]], according |
| + | to [[Planck's constant|Planck's formula]]). [[Scattering theory]] provides the theoretical | ||
| + | background for spectroscopy. | ||
| + | The probing signal used in spectroscopy can be an [[electromagnetic wave]] or a beam of | ||
| + | [[Elementary particle|particle]]s ([[electron]]s, [[positron]]s, etc.) The molecular | ||
| + | response can consist of signal absorption ([[absorption spectroscopy]]), the emission of | ||
| + | another signal ([[emission spectroscopy]]), fragmentation, or chemical changes. Spectroscopy is recognized as a powerful tool in investigating the [[microscopic]] properties of molecules, in particular their [[energy level]]s. In order to extract maximum microscopic information from experimental results, spectroscopy is often coupled with [[computational chemistry|chemical computations]]. | ||
| − | + | == History == | |
| − | + | Although the concept of molecules was first introduced in [[1811]] by [[Amadeo Avogadro|Avogadro]], and was accepted by many chemists as a result of [[John Dalton|Dalton's]] laws of Definite and Multiple Proportions (1803-1808), with notable exceptions ([[Ludwig Boltzmann|Boltzmann]], [[James Clerk Maxwell|Maxwell]], [[Willard Gibbs|Gibbs]]), the existence of molecules as anything other than convenient mathematical constructs was still an open debate in the physics community until the work of [[Jean Perrin|Perrin]] ([[1911]]), and was strenuously resisted by early [[logical positivism|positivists]] such as [[Ernst Mach|Mach]]. The modern theory of molecules makes great use of the many numerical techniques offered by [[computational chemistry]]. Dozens of molecules have now been identified in [[interstellar medium|interstellar space]] by [[rotational spectroscopy|microwave spectroscopy]]. | |
| − | |||
==References== | ==References== | ||
Revision as of 14:44, 17 May 2006
In chemistry, a molecule is an aggregate of at least two atoms in a definite arrangement held together by special forces.[1] Generally, a molecule is considered the smallest [[list of particles#Molecules|particle]] of a pure chemical substance that still retains its composition and chemical properties.[2] A molecule may be composed of the same element or atoms of two or more elements joined in a fixed ratio.[3] In the molecular sciences, a molecule is a sufficiently stable, electrically neutral entity composed of two or more atoms.[4] Molecules can be monatomic, diatomic or polyatomic. The concept of "monatomic molecule", i.e. a single-atom as found in noble gases, is used almost exclusively in the kinetic theory of gases.[5] Exactly two atoms make up diatomic molecules. Examples include O2, N2, Cl2. When diatomic The elements of Group 7A in the periodic table, the halogens, exist as diatomic elements. Diatomic molecules of two different elements are polar molecules. This is due to the difference in electronegativity - the atom with the higher electronegativity pulls the more negatively charged electrons closer to it. The higher concentration of electrons towards one side of the molecule creates a negative charge on that side and a positive charge on the other end. On the other hand, diatomic molecules of like elements have the same electronegativity and are thus nonpolar.
Polyatomic molecules contain two or more atoms. H2O and NH3 are some examples.
Empirical formula
- See main article empirical formula
The empirical formula of a molecule is the simplest integer ratio of the chemical elements that constitute the compound. For example, in their pure forms, water is always composed of a 2:1 ratio of hydrogen to oxygen, and ethyl alcohol or ethanol is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio. However, this does not determine the kind of molecule uniquely - dimethyl ether has the same ratio as ethanol, for instance. Molecules with the same atoms in different arrangements are called isomers. The empirical formula is often the same as the molecular formula but not always. For example the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
Chemical formula
- See main article chemical formula
The chemical formula reflects the exact number of atoms that compose a molecule.
Chemical bond
- See main article chemical bond
In a molecule, the atoms are joined by shared pairs of electrons in a chemical bond. It may consist of atoms of the same chemical element, as with oxygen (O2), or of different elements, as with water (H2O).
Size
Most molecules are much too small to be seen with the naked eye, but there are exceptions. DNA, a macromolecule, can reach macroscopic sizes. The smallest molecule is the hydrogen molecule. The interatomic distance is 0.15 nanometres (1.5 Å). But the size of its electron cloud is difficult to define precisely. Under standard conditions molecules have a dimension of a few to several dozen Å.
Molecular mass
- See main article molecular mass
The molecular mass can be calculated from the chemical formula and is expressed in conventional units equal to 1/12 from the mass of a 12C isotope atom. For network solids, the term formula unit is used in stoichiometric calculations.
Molecular Models
Molecular geometry
- See main article molecular geometry
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity. Isomers share a chemical formula but normally have very different properties because of their different structures. Stereoisomers, a particular type of isomers, may have very similar physico-chemical properties and at the same time very different biochemical activities.
Molecular physics
The science of molecules is called molecular chemistry or molecular physics, depending on the focus. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable system (bound state) comprising two or more atoms. The term unstable molecule is used for very reactive species, i.e., short-lived assemblies (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, [[Van der Waals bonding|Van der Waals complex]]es, or systems of colliding atoms as in [[Bose-Einstein condensate]]s. A peculiar use of the term molecular is as a synonym to covalent, which arises from the fact that, unlike molecular covalent compounds, ionic compounds do not yield well-defined smallest particles that would be consistent with the definition above. No typical "smallest particle" can be defined for covalent crystals, or network solids, which are composed of repeating unit cells that extend indefinitely either in a plane (such as in graphite) three-dimensionally (such as in diamond).
Molecular spectroscopy
Molecular spectroscopy deals with the response (spectrum) of molecules interacting with probing signals of known energy (or frequency, according to Planck's formula). Scattering theory provides the theoretical background for spectroscopy. The probing signal used in spectroscopy can be an electromagnetic wave or a beam of particles (electrons, positrons, etc.) The molecular response can consist of signal absorption (absorption spectroscopy), the emission of another signal (emission spectroscopy), fragmentation, or chemical changes. Spectroscopy is recognized as a powerful tool in investigating the microscopic properties of molecules, in particular their energy levels. In order to extract maximum microscopic information from experimental results, spectroscopy is often coupled with chemical computations.
History
Although the concept of molecules was first introduced in 1811 by Avogadro, and was accepted by many chemists as a result of Dalton's laws of Definite and Multiple Proportions (1803-1808), with notable exceptions (Boltzmann, Maxwell, Gibbs), the existence of molecules as anything other than convenient mathematical constructs was still an open debate in the physics community until the work of Perrin (1911), and was strenuously resisted by early positivists such as Mach. The modern theory of molecules makes great use of the many numerical techniques offered by computational chemistry. Dozens of molecules have now been identified in interstellar space by microwave spectroscopy.
ReferencesISBN links support NWE through referral fees
- ↑ Chang, Raymond (1998). Chemistry, 6th Ed.. New York: McGraw Hill. ISBN 0071152210.
- ↑ http://antoine.frostburg.edu/chem/senese/101/glossary/m.shtml#molecule Molecule Definition]
- ↑ Chang, Raymond (1998). Chemistry, 6th Ed.. New York: McGraw Hill. ISBN 0071152210.
- ↑ http://www.iupac.org/goldbook/M04002.pdf IUPAC Defintion of Molecule]
- ↑ http://www.ac.wwu.edu/~vawter/PhysicsNet/Topics/Thermal/kinTheoryGas.html] [1] [2]
See also
- Covalent bond
- Diatomic molecule
- Molecular geometry
- Molecular orbital
- Nonpolar molecule
- Polar molecule
Related lists
- For a list of molecules see the List of compounds
- List of molecules in interstellar space
| Particles in physics - composite particles |
| Hadrons: Baryons (list) | Mesons (list)
Baryons: Nucleons | Hyperons | Exotic baryons | Pentaquarks
|
af:Molekule
als:Molekül
ar:جزيء
bg:Молекула
ca:Molècula
cs:Molekula
da:Molekyle
de:Molekül
et:Molekul
el:Μόριο
es:Molécula
eo:Molekulo
fo:Mýl
fr:Molécule
gl:Molécula
ko:분자
hr:Molekula
io:Molekulo
id:Molekul
is:Sameind
it:Molecola
he:מולקולה
lv:Molekula
lt:Molekulė
hu:Molekula
mk:Молекула
nl:Molecuul
ja:分子
no:Molekyl
nn:Molekyl
nds:Molekül
pl:Cząsteczka
pt:Molécula
ru:Молекула
simple:Molecule
sk:Molekula
sl:Molekula
sr:Молекул
sh:Molekula
su:Molekul
fi:Molekyyli
sv:Molekyl
tl:Molekula
th:โมเลกุล
vi:Phân tử
tr:Molekül
uk:Молекула
zh:分子
| Particles in physics - composite particles |
| Hadrons: Baryons (list) | Mesons (list)
Baryons: Nucleons | Hyperons | Exotic baryons | Pentaquarks
|
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.